The agent (CG0070) yielded an overall 12-month complete response (CR) rate of 30% in a phase 2 single-arm trial in patients with BCG-unresponsive, high-grade Ta, T1, or CIS ± Ta/T1 non-muscle-invasive bladder cancer…
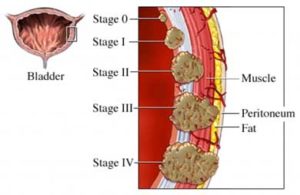
According to research the key to bladder cancer is the stage at diagnosis. If the cancer is diagnosed within the bladder (non-muscle invasive) the five year average survival rate is 70%. Muscle-invasive bladder cancer, when the cancer is diagnosed outside of the bladder brings an average five year survival rate of less than 5%.
Experiencer has taught me that while your stage at diagnosis is important, your therapy plan is even more important. Consider therapies that are outside the conventional oncology box.
Consider therapies to manage your cancer while managing your side effects aka toxicity at the same time.
In addition to the issue outlined above, many BC survivors either do not respond to BCG therapy or relapse after BCG therapy. The therapy discussed below is a new therapy for non-muscle invasive bladder cance
Once conventional treatment has been completed, either BCG or the therapy discussed below, consider evidence-based non-toxic therapies shown to reduce the risk of bladder cancer relapse. From curcumin to anti-angiogenic nutrition to frequent, moderate exercise, BC survivors can improve their prognosis.
To Learn More about Bladder Cancer and BCG therapy read the posts below-
Have you been diagnosed with bladder cancer? What stage? What therapies are you considering? Scroll down the page, post a question or comment and I will reply to you ASAP.
Thank you,
David Emerson
- Cancer Survivor
- Cancer Coach
- Director PeopleBeatingCancer
Recommended Reading:
“With dozens of clinical trials underway, there’s a race to develop, test, and receive approval for the first drug since 1998 for the treatment of high-risk bladder cancer that is unresponsive to bacillus Calmette-Guérin (BCG)…
The agent (CG0070) yielded an overall 12-month complete response (CR) rate of 30% in a phase 2 single-arm trial in patients with BCG-unresponsive, high-grade Ta, T1, or CIS ± Ta/T1 non-muscle-invasive bladder cancer…
CR was defined as the absence of evidence of disease on cystoscopy, cytology, and/or random biopsies…
He explained that the last drug to be approved by the FDA for these patients was valrubicin (Valstar, Endo Pharmaceuticals), in 1998. However, valrubicin is associated with only an 18% CR rate at 6 months. “The response is not very durable, so newer drugs are needed,” said Lee.
Intravesical gemcitabine (Gemzar, Lilly) is also an option, he commented. But both Lee and trial investigator Packiam emphasized that new intravesical agents, which are administered directly into the bladder through a catheter, and systemic immunotherapies (anti-PD-1 or anti-PD-L1 antibodies) could represent important new therapies for this disease.
CG0070 is a selective oncolytic adenovirus that leads to selective expression of granulocyte-macrophage colony-stimulating factor (GM-CSF) in retinoblastoma pathway–defective cells that are found in many tumors. It stimulates the expression of GM-CSF and has a direct oncolytic effect after local treatment (ie, intravesical administration), and it may also induce systemic, tumor-specific immunity (Clin Cancer Res. 2006;12:305-313).
After Failure of BCG
Currently, BCG is the main intravesical immunotherapy for early-stage bladder cancer.
In the current trial, all patients (n = 67) had experienced disease progression while receiving BCG therapy: they were either unable to achieve a disease-free state at 6 months after adequate BCG therapy (BCG-refractory), or they experienced recurrence after CR with BCG therapy (BCG-relapsed).
All of the patients refused the next treatment option, cystectomy or surgical removal of the bladder, and opted instead to take part in the clinical trial.
Ultimately, 10 of the 61 patients who were included in interim analysis underwent cystectomy. Pathology assessment revealed that six patients had muscle-invasive disease, not the less threatening non-muscle-invasive cancer, as first indicated.
These findings clearly illustrate the need for more treatment options, interjected Lee.
“This non-muscle-invasive disease has a high risk of progression to muscle-invasive disease, and we know that bladder cancer is very commonly understaged. So we have a disease in which clinical staging is often inaccurate, progression rates are high, and available therapies for noncystectomy candidates have low durable complete response rates, all of which point to the need for more therapeutic options,” he said…
In his presentation, Packiam highlighted that CG0070 also yielded a CR rate of 27% in patients with carcinoma in situ–containing tumors and in 48% in patients with refractory disease. These are interim results, as the trial is ongoing.
No patients with T1/CIS or Ta/CIS (n = 6) had 12-month CR. Among BCG-relapsed patients, 19% (n = 31) had a 12-month CR.
Treatment-related adverse events at 12 months included influenza-type illness (7%), fatigue (4%), and chills (1%). Five deaths occurred secondary to progressive urothelial carcinoma, esophageal carcinoma, lung carcinoma, and cardiac disease.”
The study was funded by Cold Genesys, the maker of CG0070. Dr Packiam and Dr Lee have disclosed no relevant financial relationships.
Curcumin, Green Tea Extract Enhance BCG for Bladder Cancer
In a syngeneic bladder cancer model, curcumin alone reduced the bladder tumor volume, but a significantly greater reduction was observed when BCG and curcumin were used in combination…
He is starting BCG (
Bacillus Calmette-Guerin) intravesical immunotherapy on 9/10/18 for the next 6 weeks. What is your recommended dosage/protocol for taking green tea and green tea extract?
Michele
Hi Michele,
I am sorry to read of your husband’s bladder cancer diagnosis though glad to read that there is no evidence of “invasion.”
Two things. In answer to your question about green tea extract dosing, the study that you came in on talks about time and dose dependant manner as well as different types of Bladder Cancer cells so there is no specific dose given for your situation. Though my cancer is different and I have been in remission for years, I also supplement with green tea extract. I follow the dose given on the label- 750 mg in the case of the brand linked in that study.
The other issue I wanted to mention are other evidence-based, non-toxic supplements that integrate or synergize with BCM to enhance the efficacy of this therapy. An antioxidant, angiogenic supplement called curcumin is cytotoxic to bladder cancer and according to the study below, enhances the efficacy of Bacillus Calmette-Guerin.
Your challenge is that curcumin is known to be difficult for the body to ingest. Therefore please read about bioavailable curcumin formulas.
Recommended Reading:
“Although Bacillus Calmette-Guerin (BCG) intravesical therapy is a standard treatment for bladder cancer, eventual failure of response is a major problem. Treatments that can augment BCG therapy are urgently needed. We investigated whether curcumin, a component of Curcuma longa (also called turmeric), has potential to improve the current therapy using in vitro and in vivo MBT-2 murine tumor models.
We found that curcumin potentiated BCG-induced apoptosis of human bladder cancer cells. BCG stimulated the release of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) from peripheral mononuclear neutrophils in a dose- and time-dependent manner, whereas curcumin enhanced the upregulation of TRAIL receptors.
In a syngeneic bladder cancer model, curcumin alone reduced the bladder tumor volume, but a significantly greater reduction was observed when BCG and curcumin were used in combination…
Overall, our results suggest that curcumin potentiates the antitumor effect of BCG through the inhibition of NF-kappaB and induction of TRAIL receptors in bladder cancer cells.”
The Most BioAvailable Curcumin Forumula
“Based on a review of these studies, it is evident that better bioavailability of formulated curcumin (CU) products is mostly attributed to improved solubility, stability, and possibly low first-pass metabolism”
A search of the Pubmed database for the word curcumin yields 601 studies spaning health topics from multiple myeloma and colorectal cancer, to chemotherapies that synergizes with CU, to Alzheimer’s Disease, arthritis and more. Based on years of reading studies and personal accounts, I think it is safe to say that CU supplementation is safe and relatively inexpensive.

I have read about myeloma patients taking daily doses of CU from 400 milligrams to 8 grams (1000 milligrams = 1 gram). By almost any measure, CU is a safe, inexpensive wonder drug.
The only challenge is that CU is famously difficult to absorb in the body. In other words, a person has to mix curcumin with some sort of fat (coconut oil, chocolate, etc.) or take a brand of curcumin capsule that is already formulated to be more “bioavailable” in order to derive the full benefit of CU.
The study linked and exerpted below reviews different formulations of CU. The study itself lists the three most bioavailable formulation/brand of CU and I’ve added an excerpt from a further review from Consumerlab.com that lists four additional bioavailable brands of CU.
Recommended Reading:
“CU is a bright yellow chemical produced by some plants. It is the principal curcuminoid of turmeric (Curcuma longa), a member of the ginger family, Zingiberaceae. It is sold as an herbal supplement, cosmetics ingredient, food flavoring, and food coloring.[1]“
“Curcumin is a widely studied natural compound which has shown tremendous in vitro therapeutic potential. Despite that, the clinical efficacy of the native CU is weak due to its low bioavailability and high metabolism in the gastrointestinal tract. During the last decade, researchers have come up with different formulations with a focus on improving the bioavailability of curcumin. As a result, a significant number of bioavailable curcumin-based formulations were introduced with the varying range of enhanced bioavailability.
The purpose of this review is to collate the published clinical studies of CU products with improved bioavailability over conventional (unformulated) CU. Based on the literature search, 11 curcumin formulations with available human bioavailability and pharmacokinetics data were included in this review. Further, the data on clinical study design, analytical method, pharmacokinetic parameters and other relevant details of each formulation were extracted.
Based on a review of these studies, it is evident that better bioavailability of formulated curcumin products is mostly attributed to improved solubility, stability, and possibly low first-pass metabolism. The review hopes to provide a quick reference guide for anyone looking information on these bioavailable curcumin formulations.
Based on the published reports,
exhibited over 100-fold higher bioavailability relative to reference unformulated CU. Suggested mechanisms accounting for improved bioavailability of the formulations and details on the bioanalysis methods are also discussed.”
According to Consumerlab.com:
“Novasol has the highest bioavailability (185 x compared to unforumulated CU), followed by Curcuwin (136 x), Longvida (100 x), Meriva (48 x), BCM-95 (27 x), Curcumin C3 Complex + Bioperene (20 x), and then Theracumin (16 x).”