- You are here:
- Home »
- Blog »
- Uncategorized »
- Cancer Coaching- Breast Cancer + Aromatase Inhibitor…Polyp
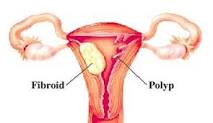
Cancer Coaching- Breast Cancer + Aromatase Inhibitor…Polyp

“In standard dosages, tamoxifen may be associated with endometrial proliferation, hyperplasia, polyp formation, invasive carcinoma, and uterine sarcoma.”
Dear Cancer Coach- I am a breast cancer survivor and was on tamoxifen for five years. A polyp has just been discovered in my uterus. I am to have an MRI and most likely a biopsy.
My question is: due to the tamoxifen is there any chance of the polyp being benign?
Hi BC survivor-
I am sorry to read of your breast cancer diagnosis however it sounds as though you in remission. I have excerpted several paragraphs from studies that I think speak to your question “due to the tamoxifen is there any chance of the polyp being benign?”
If I understand the studies, if your tamoxifen dose is standard, the polyp is likely to be benign and a small risk that the polyp is cancerous. I would proceed with both your MRI and a biopsy.
Let me know if you have any questions or concerns.
Good luck-
David Emerson
- Cancer Survivor
- Cancer Coach
- Director PeopleBeatingCancer
Tamoxifen and Uterine Cancer
“Uterine sarcomas consisting of leiomyosarcoma, carcinosarcoma, high-grade endometrial stromal sarcoma, adenosarcoma, and sarcoma not otherwise specified, are rare and estimated to comprise 8% of all invasive uterine cancer cases (8). In a review of all National Surgical Adjuvant Breast and Bowel Project breast cancer treatment trials, the rate of sarcoma in women treated with tamoxifen was 17 per 100,000 patient years versus none in the placebo group (7).”
“In standard dosages, tamoxifen may be associated with endometrial proliferation, hyperplasia, polyp formation, invasive carcinoma, and uterine sarcoma.
Most studies have found that the increased relative risk of developing endometrial cancer for women taking tamoxifen is two to three times higher than that of an age-matched population (1–3). The level of risk of endometrial cancer in women treated with tamoxifen is dose and time dependent. Studies suggest that the stage, grade, histology, and biology of tumors that develop in individuals treated with tamoxifen (20 mg/d) are no different from those that arise in the general population (3, 4). However, some reports have indicated that women treated with a higher dosage of tamoxifen (40 mg/d) are more prone to develop more biologically aggressive tumors (5).”
“Gibson et al [19] recently reported the results of a retrospective review of endometrial pathology found at dilatation and curettage (D&C) performed in 240 breast cancer patients. Seventy-five (31%) of these patients had received tamoxifen, 20 mg/day, for a mean duration of 26 months as therapy for their breast cancer. Patients in this study were stratified as symptomatic (abnormal bleeding) or asymptomatic (curettage performed as part of another procedure or due to the presence of a thickened endometrial stripe on ultrasound examination).
Among tamoxifen users who were symptomatic at the time of D&C, the incidence of endometrial polyps was 15%; hyperplasia, 2%; and adenocarcinoma, 11%; compared with an incidence of 13%, 4%, and 11%, respectively, for nonusers. Among asympto- matic patients receiving tamoxifen, the incidence of polyps, hyperplasia, and adenocarcinoma was 9%, 0%, and 0%, while nonusers had an incidence of 5%, 4%, and 0%, respectively. All of the cases of endometrial carcinoma were associated with abnormal vaginal bleeding. The 11% incidence of endometrial carcinoma in this study was comparable to what has been reported for all patients presenting with postmenopausal bleeding in the general population [20].
The mean duration of tamoxifen use in those patients found to have adenocarcinoma was 44 months, compared with 18 months in those patients with polyps, and 19 months in the patient with hyperplasia. This difference was not statistically significant, possibly due to small numbers. The authors concluded that short-term tamoxifen use at the 20 mg/day dosage level was not associated with a higher incidence of abnormal pathology, as detected at the time of D&C in breast cancer patients.”