Leave a Comment:
1 comment
[…] For Bladder Cancer Cystectomy, Less Is More […]
Reply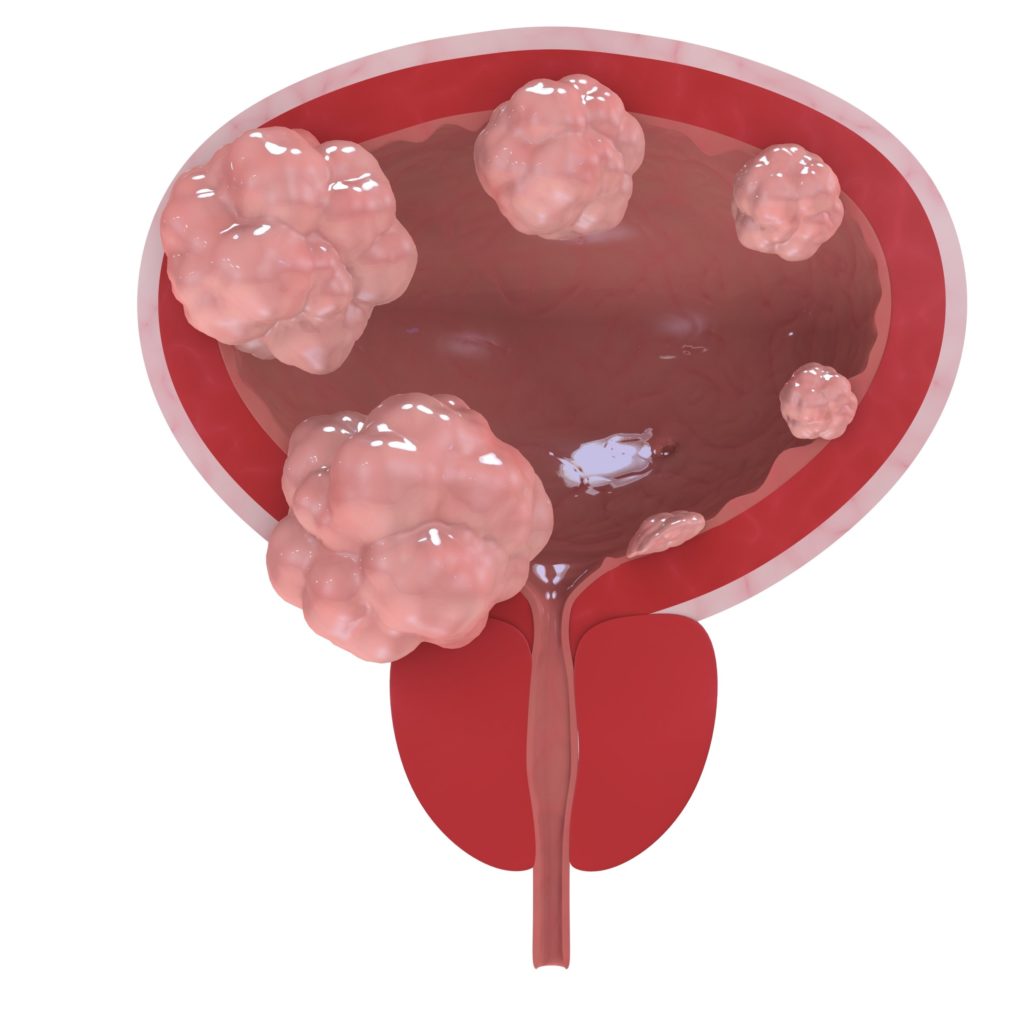
The article linked and excerpted below has a lot to say…to patients and survivors of many different types of cancers. But because the article provides the most actionable information for bladder cancer patients, I’ve chosen to focus specifically on patient who have been diagnosed with “muscle-invasive bladder cancer.”
The bottom line below is that conventional oncology continues to discover new therapies to do less…giving the patient more. In the case below, patients with muscle-invasive bladder cancer may be eligable to keep part of their bladder and lead a more normal life.
I believe it is critical to add the idea of evidence-based, non-toxic therapies to reduce the risk of bladder cancer relapse. Therapies such as curcumin have been shown to reduce the risk of BC relapse.
Please scroll down the page to learn about the most bioavailable curcumin formulas.
Have you been diagnosed with bladder cancer? Scroll down the page, post a question or comment and I will reply to you ASAP.
Thank you,
David Emerson
“Muscle invasive bladder cancer (MIBC) is a cancer that spreads into the detrusor muscle of the bladder. The detrusor muscle is the thick muscle deep in the bladder wall. This cancer is more likely to spread to other parts of the body. About 1 out of 4 people who get bladder cancer in the United States have the muscle invasive kind…”
“Tom Maguire always figured that, if he ever developed cancer, he would pursue the toughest treatment available. “You destroy yourself, and then you can come back,” he said.
His view was tested earlier this year when he was diagnosed with bladder cancer that had invaded the muscle wall of the organ. The standard of care, he learned, usually involves removing the bladder. He would have the choice of permanently wearing a bag to collect his urine or having a difficult surgery to fashion a new bladder from his intestines. Both prospects filled the 63-year-old avid hiker and scuba diver with dread.
Then doctors at Philadelphia’s Fox Chase Cancer Center told him about a new clinical trial designed to allow people with certain types of tumors to keep their bladders while being closely monitored.
Since getting into the trial a few months ago, “I have been walking on air,” he said. “I guess you don’t always have to take an all-in, nuclear approach…”
But today, the “fighting cancer patient” metaphor is falling out of favor, not only because it subtly blames patients who “lose the fight” but also because it doesn’t capture a world of new biological insights, improved treatments and molecular tests that are transforming how cancer is treated.
At the root of the change is the recognition that not all cancers are the same: Some need to be bludgeoned, but others can be treated with more tailored therapies or simply watched. Equipped with new tools and evidence, oncologists are “de-escalating” — cutting back on toxic and costly approaches likely to do more harm than good. “Knowing when not to treat is great medicine,” Bekelman said…
“It’s a precision oncology story,” said Norman “Ned” Sharpless, the director of the National Cancer Institute. “Some people will benefit from chemotherapy, and some won’t.” As more molecular tests come online, he said, they will be increasingly helpful in sorting cancers and patients into the right groups…
That’s why biomarkers are so important to help guide therapy decisions and build acceptance for them. In 2015, Plimack and her colleagues discovered that certain genetic mutations in bladder-cancer tumors predict that chemotherapy will wipe out the cancer and make it unlikely to recur.
These biomarkers, which occur in only a minority of patients, suggest that treatment can be based partly on patients’ individual risks of recurrence, said Daniel Geynisman, the Fox Chase oncologist leading the bladder-cancer trial. “One approach doesn’t fit everybody,” he said. The way medicine is currently practiced, “we are clearly overtreating a lot of patients, and we are probably undertreating some patients.”
Bladder cancer is the sixth-most common cancer in the United States, according to the National Cancer Institute. About 81,000 people are expected to be diagnosed with the disease this year, and more than 17,000 will die. Many of the cases are cancers in the lining of the bladder that have not invaded the muscle wall; the tumors are removed, repeatedly if necessary. But cancer that has grown into the muscle wall of the bladder is considered invasive, with the potential to spread to other organs, making the disease much harder to treat.
In most of those cases, the current standard of care involves extensive surgery to remove the bladder and prostate in men and the bladder and uterus in women. “Nobody wants that surgery,” Geynisman said.
Fox Chase isn’t alone in testing the concept; other trials using biomarkers to try to preserve bladders are getting underway at other medical centers, Geynisman noted. If the new techniques work, and are confirmed in larger trials, it will change the traditional standard of care, he said. “And that,” he added, “will be a major de-escalation.”
“Based on a review of these studies, it is evident that better bioavailability of formulated curcumin (CU) products is mostly attributed to improved solubility, stability, and possibly low first-pass metabolism”
A search of the Pubmed database for the word curcumin yields 601 studies spaning health topics from multiple myeloma and colorectal cancer, to chemotherapies that synergizes with CU, to Alzheimer’s Disease, arthritis and more. Based on years of reading studies and personal accounts, I think it is safe to say that CU supplementation is safe and relatively inexpensive.
I have read about myeloma patients taking daily doses of CU from 400 milligrams to 8 grams (1000 milligrams = 1 gram). By almost any measure, CU is a safe, inexpensive wonder drug.
The only challenge is that CU is famously difficult to absorb in the body. In other words, a person has to mix curcumin with some sort of fat (coconut oil, chocolate, etc.) or take a brand of curcumin capsule that is already formulated to be more “bioavailable” in order to derive the full benefit of CU.
The study linked and exerpted below reviews different formulations of CU. The study itself lists the three most bioavailable formulation/brand of CU and I’ve added an excerpt from a further review from Consumerlab.com that lists four additional bioavailable brands of CU.
“CU is a bright yellow chemical produced by some plants. It is the principal curcuminoid of turmeric (Curcuma longa), a member of the ginger family, Zingiberaceae. It is sold as an herbal supplement, cosmetics ingredient, food flavoring, and food coloring.[1]“
“Curcumin is a widely studied natural compound which has shown tremendous in vitro therapeutic potential. Despite that, the clinical efficacy of the native CU is weak due to its low bioavailability and high metabolism in the gastrointestinal tract. During the last decade, researchers have come up with different formulations with a focus on improving the bioavailability of curcumin. As a result, a significant number of bioavailable curcumin-based formulations were introduced with the varying range of enhanced bioavailability.
The purpose of this review is to collate the published clinical studies of CU products with improved bioavailability over conventional (unformulated) CU. Based on the literature search, 11 curcumin formulations with available human bioavailability and pharmacokinetics data were included in this review. Further, the data on clinical study design, analytical method, pharmacokinetic parameters and other relevant details of each formulation were extracted.
Based on a review of these studies, it is evident that better bioavailability of formulated curcumin products is mostly attributed to improved solubility, stability, and possibly low first-pass metabolism. The review hopes to provide a quick reference guide for anyone looking information on these bioavailable curcumin formulations.
Based on the published reports,
exhibited over 100-fold higher bioavailability relative to reference unformulated CU. Suggested mechanisms accounting for improved bioavailability of the formulations and details on the bioanalysis methods are also discussed.”
According to Consumerlab.com:
“Novasol has the highest bioavailability (185 x compared to unforumulated CU), followed by Curcuwin (136 x), Longvida (100 x), Meriva (48 x), BCM-95 (27 x), Curcumin C3 Complex + Bioperene (20 x), and then Theracumin (16 x).”
[…] For Bladder Cancer Cystectomy, Less Is More […]
Reply