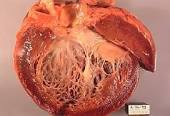
Cardiovascular immune-related adverse events (cardiotoxicity of CTLA-4 antagonists, PD-1 inhibitors), particularly myocarditis, are increasingly recognized.
I underwent adriamycin, cytoxan, melphalan and busulphan in the span of 9 months in 1995. I was diagnosed with chemotherapy-induced cardiomyopathy and chronic atrial fibrillation fifteen years later.
I have come to believe that chemotherapy-induced cardiotoxicity is the most damaging short, long-term and late stage side effect of conventional oncology causing death and disability, potentially for the entire life of the cancer survivor.
The goal of the first study linked and excerpted below is to identify new diagnoses of various heart failure occurrences “after therapy onset” caused by immune checkpoint inhibitors.
When I read this I couldn’t help asking myself if oncology (much less cardio-oncology) has learned nothing from decades of cardio-toxic chemotherapy regimens such as
- Anthracyclines
- 5-FU,
- Capecitabine
- Melphalan
- Cytoxan
- Busulphan
- paclitaxel,
- docetaxel,
- cabazitaxel
- Trastuzumab
- Pertuzumab
- Bevacizumab
- Sunitinib
- Sorafenib
- Nilotinib
- Dasatinib
- Imatinib
As well as many other chemo regiments. By exclaiming “have they learned nothing?! I am trying to draw attention to the fact that oncology has known of the toll cardio-toxic therapies have taken on cancer patients for decades now. Oncology has identified both conventional as well as evidence-based non-conventional therapies shown to reduce or even prevent cardio-toxic affects.
And most importantly, oncology knows that the damage caused by cardio-toxic therapy can show-up years after administering these drugs. And yet the researchers of both studies below ignored all of that.
If you are considering undergoing or have already undergone a cardio-toxic therapy- either a conventional chemotherapy drug or an immune checkpoint, consider learning about therapies, both conventional and non, shown to protect or heal your heart.
If you’d like to learn more about cardiotoxicity and how to manage or prevent this debilitating side effect, scroll down the page, post a question or comment and I will reply to you ASAP.
Thank you,
David Emerson
- Cancer Survivor
- Cancer Coach
- Director PeopleBeatingCancer
Recommended Reading:
PeopleBeatingCancer Side Effects Program
“The cardiotoxic potential of these agents was first described in murine models and, more recently, in numerous clinical case reports of pericarditis, myocarditis, pericardial effusion, cardiomyopathy, and new arrhythmias. The objective of our study was to determine the frequency of and associated risk factors for cardiotoxic events in patients treated with immune checkpoint inhibitors…
Medical records of patients who underwent immunotherapy with:
- durvalumab,
- ipilimumab,
- nivolumab,
- and pembrolizumab
at Wake Forest Baptist Health were reviewed. We collected retrospective data regarding sex, cancer type, age, and cardiovascular disease risk factors and medications.
We aimed to identify new diagnoses of
- heart failure,
- atrial fibrillation,
- ventricular fibrillation/tachycardia,
- myocarditis,
- and pericarditis
after therapy onset…
“The use of immune checkpoint inhibitors (ICIs) in cancer management has significantly increased in recent years. The remarkable efficacy of these agents as anti-neoplastic therapies is shadowed by their potential for inducing autoimmune or inflammatory side effects, collectively termed immune-related adverse events. Cardiovascular immune-related adverse events, particularly myocarditis, are increasingly recognized. The prevalence of myocarditis has been reported from 0.06% to 2.4%, with a higher risk in combination immunotherapy.1-3…
Based on recent data, ICI-induced myocarditis can no longer be considered a rare immune-related adverse event.4 Other manifestations include:
- pericardial disease,6
- vasculitis,5
- Takotsubo syndrome,7
- destabilization of atherosclerotic lesions,8
- venous thromboembolism,9 or c
- onduction abnormalities,4
but epidemiological data are sparse.
Here we summarize the current approach to the diagnosis and management of ICI-associated myocarditis…
Cardiac immune-related adverse events appear more frequently in patients treated with CTLA-4 antagonists compared with PD-1 inhibitors,12 and the risk increases with combination therapy.3,13,14 The development of cardiac immune-related adverse events in patients treated with combination therapy leads to ICI discontinuation in up to 50% of patients.13,14—
CTLA-4 is expressed predominantly on T-cells, and PD-1 may also be expressed on human hearts.15 CTLA-4 and PD-1-deficient mice develop autoimmune myocarditis or dilated cardiomyopathy, eventually leading to death due to heart failure.15,16…
Diagnosis of ICI-Associated Myocarditis
The onset of ICI-associated myocarditis is within 3 months of treatment initiation in 81% of cases,2,3 with a median time of 17-65 days after the first dose of ICI.20 Symptoms are non-specific and may include
- dyspnea,
- chest pain,
- fatigue,
- myalgia,
- palpitations,
- syncope,
- dizziness, or
- altered mental status.
Subclinical myocarditis has also been described, posing management challenges.21…
It is unclear whether baseline ECG or troponins predict ICI-associated myocarditis or modify management/outcomes. Echocardiography is useful for determining cardiac function, but the definitive imaging modality is cardiac magnetic resonance (CMR), which allows for better tissue characterization…
Management of ICI-Associated Myocarditis
The American Society of Clinical Oncology recently issued a practice guideline for the management of ICI-associated myocarditis.9 Baseline ECG and serum troponins are recommended. In all cases suspected of ICI-induced cardiotoxicity, ICI therapy should be withheld.
Serum high-sensitivity cardiac troponin T (hs-cTnT) and brain natriuretic peptide should be measured. An ECG and a transthoracic echocardiogram should be performed. Further management is guided by symptoms and serum troponin levels, but patients should be admitted.
In symptomatic cases, CMR and cardiac catheterization with EMB are warranted. In asymptomatic cases, we recommend CMR if hs-cTnT is >100 ng/L with no other identifiable cause; cardiac catheterization with EMB should be added if hs-cTnT is >250 ng/L based on the institutional agreed practice at The University of Texas MD Anderson Cancer Center. Ongoing research is validating this approach. If myocarditis is confirmed, serum erythrocyte sedimentation rate, C-reactive protein, C3, and C4 should be measured to guide immunosuppressive therapy going forward.22
The severity of the myocarditis may be broadly classified into four grades with independent treatment strategies.9
- G1 is defined as subclinical myocarditis based on an abnormal test; it is unclear whether this grade warrants specific treatments
- Symptomatic but clinically stable cases with abnormal tests may be classified as G2 and G3; treatment should be attempted with 1-2 mg/kg prednisone or methylprednisolone
- Decompensated cases are classified as G4 and require more aggressive immunosuppressive regimens, such as 1-2 mg/kg prednisone + mycophenolate, anti-thymocyte globulin, or infliximab (to be avoided in cases of heart failure); these cases may also require advanced heart failure support
For most immune-related adverse events, cessation of therapy is recommended for only G2 or higher, but for cardiac immune-related adverse events, the consensus statements recommend stopping at G1 due to the high mortality observed in cardiac immune-related adverse events.9
Conclusion
Several important challenges in the management of ICI-associated cardiotoxicity exist. The rates of mortality and major adverse cardiovascular events remain high with current treatment strategies.2 Reports have emerged of therapeutic success with various immunosuppressive regimens, such as with intravenous immunoglobulins, but no clinical trials comparing the efficiency of these regimens currently exist.2
Further research is warranted to evaluate response to increased doses of glucocorticoids and varying immunosuppression regimens. The benefit of EMB on all patients with suspicion of ICI-associated cardiotoxicity in cases with negative CMR is not currently well-established. Furthermore, the risks versus benefits of cardiac catheterization and EMB in high-risk patients (e.g., with thrombocytopenia) are also unclear.”