Recently Diagnosed or Relapsed? Stop Looking For a Miracle Cure, and Use Evidence-Based Therapies To Enhance Your Treatment and Prolong Your Remission
Multiple Myeloma an incurable disease, but I have spent the last 25 years in remission using a blend of conventional oncology and evidence-based nutrition, supplementation, and lifestyle therapies from peer-reviewed studies that your oncologist probably hasn't told you about.
Click the orange button to the right to learn more about what you can start doing today.
- You are here:
- Home »
- Blog »
- Multiple Myeloma »
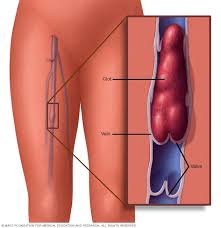
- Multiple Myeloma Side Effects- Chemo & Blood Clot (VTE) Prevention
Multiple Myeloma Side Effects- Chemo & Blood Clot (VTE) Prevention

Although anticoagulant therapy is recommended to prevent VTE, the increased risk of anticoagulant-induced bleeding is however of significant concern in patients with cancer…
When it comes to VTE aka blood clots, multiple myeloma (MM) patients have two strikes against them going into active therapy. MM itself can cause blood clots and the chemotherapy to treat your MM can also cause blood clots. It should come as no surprise then, that blood clots are a common multiple myeloma side effect.
The MM patient’s conventional, FDA approved choices to manage or prevent DVTs are below.
- low molecular weight heparin (LMWH)
- vitamin K antagonists (VKAs)
- direct oral anticoagulants (DOACs)
The challenge to MMers is that the evidence comparing these three therapies is conflicting- see the text highlighted in maroon below.
Further, if you’re considering lenalidomide (revlimid) chemotherapy, blood thinner prophylaxis may not help.
I developed a blood clot shortlly after beginning my induction therapy for MM back in the spring of 1995. I was put in the hospital for 4 days undergoing low molecular weight heparin (LMWH). As luck would have it, I was able to watch the March Madness tourney each and every day.
My DVT seemed to dissolve and I left the hospital. From that point on, Dr. Berger included a vitamin K antagonists (VKAs), warfarin, to my therapy regimen. I developed another DVT in the other leg about a year later. To this day I’m not sure if the second blood clot was caused by chemotherapy or my MM.
At some point since my blood clot occurrances, I settled into a long-term side effect called post-thrombotic symdrome.
Meaning I live with a little bit of DVT in both of my legs. Nothing dramatic, I just don’t stand for too long. And when I sit, I elevate my legs.
Dr. Berger wanted me to take warfarin for the rest of my life, starting back in 1995. After a year or two on warfarin (DOACs had not yet been invented) I decided that I didn’t want the short and long-term risks of daily warfarin.
I now supplement with a number of evidence-based but non-toxic blood thinning products such as omega-3 fatty acids, wobenzyme (enzymes), curcumin, vitamin E and others. I exercise moderately but frequently. I live a number of anti-blood clot lifestyle therapies.
If conventional medicince developed a therapy that thinned my blood without also risking “major bleeding events” or increased my risk of cerebral aneurysm, I would gladly take this therapy. In the meantime, I choose to take evidence-based but non-toxic therapies to reduce my risk of DVT.
To learn more about evidence-based, non-toxic therapies to reduce your risk of DVT aka blood clots, scroll down the page, post a question or comment and I will reply to you ASAP.
Thank you,
David Emerson
- MM Survivor
- MM Cancer Coach
- Director PeopleBeatingCancer
Recommended Reading:
- Resolve Blood Clots in Multiple Myeloma (MM)-
- Oncologists Don’t Explain Blood Cancer Diagnosis, Therapies or Side Effects-
- The Cancer-Suicide Link
Efficacy and Safety of Direct Oral Anticoagulants for Risk of Cancer-Associated Venous Thromboembolism
“Efficacy and safety of direct oral anticoagulants (DOACs) for preventing primary and recurrent venous thromboembolism (VTE) in patients with cancer remain unclear. In this study, we conducted a systematic review to summarize the most up-to-date evidence from randomized controlled trials (RCTs).
Our primary outcomes included
- the benefit outcome (VTE) and
- safety outcome (major bleeding)
To summarize, DOACs were found to have a favorable effect on risk of VTE but a nonsignificant higher risk of major bleeding compared with non-DOACs in patients with cancer…
Venous thromboembolism (VTE), including deep vein thrombosis (DVT) and pulmonary embolism (PE), occurs in up to 15% of patients with cancer during the course of their disease.1,2 Venous thromboembolism is found to be the second leading cause of death after malignancy itself.3 Compared with those without cancer, the risk of recurrent VTE is at least 2 fold higher in patients with cancer.4
Although anticoagulant therapy is recommended to prevent VTE, the increased risk of anticoagulant-induced bleeding is however of significant concern in patients with cancer…
Meta-analyses based on findings from randomized controlled trials (RCTs) have reported conflicting results, as follows: (1) DOACs had superior efficacy and safety over VKAs, though nonsignificantly, and (2) DOACs are equally effective and safe when compared with LMWH.7–9 Nevertheless, some studies also reported a lower risk of VTE and an increased risk of bleeding in DOACs than in VKAs or LMWH,10,11 while others indicated reduction in risk of major bleeding in DOACs.12,13…
Venous thromboembolism risk with contemporary lenalidomide-based regimens despite thromboprophylaxis in multiple myeloma: A systematic review and meta-analysis
“By analyzing evidence from relevant phase 1, 2, and 3 clinical trials identified from Ovid MEDLINE, Embase, and Cochrane databases, researchers estimated the incidence of venous thromboembolism (VTE) among patients with myeloma who have already received thromboprophylaxis with currently used lenalidomide-based regimens.
The included studies were those that assessed lenalidomide-based regimens with thromboprophylaxis. Overall 1,372 citations were identified, including 51 clinical trials and 9,069 patients examined. Aspirin, low-molecular-weight heparin or warfarin constituted the most commonly used thromboprophylaxis agents.
In trials involving patients who had newly diagnosed and relapsed/refractory myeloma, the pooled incidence of VTE was estimated to be 6.2% over median treatment durations ranging from 2 to 34 cycles, which translated into 1.2 VTE events per 100 patient-cycles.
Overall, a substantial risk of VTE associated with lenalidomide-based regimens was evident in controlled clinical trial settings, despite adequate thromboprophylaxis.”