Recently Diagnosed or Relapsed? Stop Looking For a Miracle Cure, and Use Evidence-Based Therapies To Enhance Your Treatment and Prolong Your Remission
Multiple Myeloma an incurable disease, but I have spent the last 25 years in remission using a blend of conventional oncology and evidence-based nutrition, supplementation, and lifestyle therapies from peer-reviewed studies that your oncologist probably hasn't told you about.
Click the orange button to the right to learn more about what you can start doing today.
- You are here:
- Home »
- Blog »
- non-conventional therapies »
- Multiple Myeloma Side Effect- Blood Clot Therapies
Multiple Myeloma Side Effect- Blood Clot Therapies
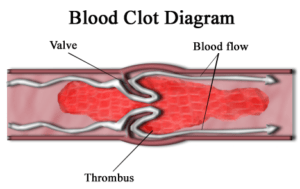
“VTE/DVT is an under-recognized but frequently encountered complication … common in patients with multiple myeloma receiving IMiDs (revlimid), and can cause disability, delay or complicate chemotherapy…
Blood clots are a common multiple myeloma side effect. Two weeks after I began induction therapy for multiple myeloma (MM), I developed a deep vein thrombosis (DVT). Blood clots are a common side effect of Revlimid (bortezomib). According to the study linked below, my SAVED score would have been 3 and therefore would have indicated that my risk of blood clot was high.
Newly diagnosed MM patients undergoing revlimid should understand that managing side effects from chemotherapy can increase their quality of life and quantity of life. Blood clots, both DVT and VTE, are a great example of a common side effect that is relatively easy to prevent.
Though the standard-of-care therapy to reduce the risk of blood clots are blood thinning medications such as Plavix, Eliquis and Xarelto, there are many evidence-based supplements and lifestyle therapies that can also reduce the risk of blood clots.
To lower my risk of blood clots I take:
In addition to frequent, moderate exercise and nutrition to also reduce my risk of blood clots.
Have you been diagnosed with multiple myeloma? Are you about to begin induction therapy and are wondering about your risk of DVT? Scroll down the page, post a question or comment and I will reply to ou ASAP.
Thank you,
David Emerson
- MM Survivor
- MM Cancer Coach
- Director PeopleBeatingCancer
Recommended Reading:
VTE Thromboprophylaxis Essential When Treating Newly Diagnosed MM With Lenalidomide, Steroid-Based Therapy
“Results of a recent study highlight the importance of proper thromboprophylaxis for venous thromboembolism (VTE) in patients with newly diagnosed multiple myeloma (NDMM) who are receiving lenalidomide and a steroid-based treatment…
The most common induction therapy, in 86% of patients, consisted of lenalidomide with bortezomib and low-dose dexamethasone…
VTE was reported in 9% of the patients overall, occurring in 13% of high-risk patients and 5.6% of low-risk patients. VTE occurred in 7.6% of patients who were given optimal thromboprophylaxis.
Major bleeding was reported in 1 patient taking aspirin, and 1 patient on warfarin experienced minor bleeding…”
Thromboembolic Events With Lenalidomide-based Therapy for Multiple Myeloma
“DISCUSSION-
Thrombotic complications of lenalidomide therapy have evoked a great deal of interest. The underlying mechanisms remain unclear. We performed a pooled analysis of newly diagnosed myeloma patients receiving lenalidomide as initial therapy and report a cumulative deep vein thrombosis rate of 8%.
This rate is in the lower range of what has been previously reported in the literature in this setting (Table 4). The lower rate may be related to the high percentage of our patient population (88%) who received anticoagulant prophylaxis.
In addition, 58% of patients studied were on low-dose steroids, namely, weekly dexamethasone, or prednisone. The thrombotic rate in patients receiving low-dose corticosteroids in this study was 6%.
In contrast, the thrombotic rate in patients on high-dose corticosteroids was 12% (P = .4). The difference in incidence of thrombosis based on the dose of corticosteroids did not achieve statistical significance, probably due to the low event rate and the relatively small sample size of this study. However, these data are consistent with a prior report in which the thrombosis risk with lenalidomide was significantly associated with steroid dose.17…”
Tally of Risk Factors Can Identify Myeloma Patients at Risk of Deep-vein Blood Clots, Study Suggests
“A simple score of risk factors could help alert doctors to a likelihood of dangerous blood clots, called venous thromboembolism, forming as a side effect of immunomodulatory therapies (IMiD) for multiple myeloma.
Researchers established the set of factors — based on a person’s
- age,
- race,
- and medical history —
and how to score them so to better identify patients at risk for these clots, which could be prevented with anticoagulant (blood thinner) use…
Patients are especially at risk when using immunomodulatory treatment regimens such as Revlimid (lenalidomide), Thalomid (thalidomide), and Pomalyst(pomalidomide; sold as Imnovid in Europe)…
“VTE is an under-recognized but frequently encountered complication … common in patients with multiple myeloma receiving IMiDs, and can cause disability, delay or complicate chemotherapy, and — in rare cases — be fatal…”
Researchers identified five clinical variables associated with a greater VTE risk:
- a prior history of VTE,
- surgery within 90 days,
- age 80 or older,
- high dexamethasone dose (greater than 160 mg), and
- non-Asian race…
The model identified approximately 30% patients at high risk. Depending on the dataset, these patients were 85% to 98% more likely (nearly twice as likely) compared to low-risk patients of having a VTE..”
Derivation and Validation of a Risk Assessment Model for Immunomodulatory Drug–Associated Thrombosis Among Patients With Multiple Myeloma
“Background: Although venous thromboembolism (VTE) is a significant complication for patients with multiple myeloma (MM) receiving immunomodulatory drugs (IMiDs), no validated clinical model predicts VTE in this population…
Conclusions: The SAVED score outperformed the current NCCN Guidelines in risk-stratification of patients with MM receiving IMiD therapy. This clinical model can help inform providers and patients of VTE risk before IMiD initiation and provides a simplified clinical backbone for further prognostic biomarker development in this population…”
“patients with advanced cancer admitted to specialist palliative care units (SPCUs) found that 1 in 3 had deep vein thrombosis and that thromboprophylaxis may have little benefit for late-stage disease.”
Patients with advanced end-stage multiple myeloma undergoing palliative care shouldn’t have to worry about blood clots aka DVT. According to the study linked and excerpted below, they do. Thromboprophylaxis is/are therapies to reduce the occurrence of thromboses aka blood clots.
Patients with advanced MM probably undergo high-dose chemotherapy regimens proceeding palliative care. It is pretty-well established that chemotherapy causes blood clots.
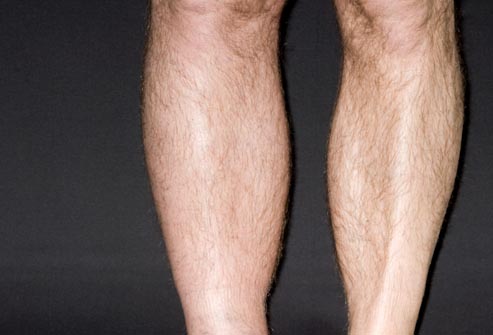
According to the article, if the patient has had previous blood clots, if he/she is bed bound or if the patient suffers from “lower limb edema” (swollen calves, ankles, etc.) then they are at a high risk of DVT.
Most importantly, thromboprophylaxic (drugs to prevent DVT), don’t help.
As both a cancer survivor and cancer coach, all I can offer is my experience as someone having had two DVT’s previously. I developed a blood clot in my left leg about two weeks after my first course of VAD induction therapy. I developed a DVT in the other leg a couple of years later.
I took and continue to take Nattokinase and Wobenzyme. Blood clot prevention will be a part of my life for the rest of my life as far as I can tell.
Recommended Reading:
- End-of-Life Mind-Body Therapy for Advanced Cancer Patients
- Resolve Blood Clots in Multiple Myeloma (MM)
- Palliative Cancer Care-Multiple Myeloma Cancer Coaching-
Thromboprophylaxis May Not Benefit Patients With Advanced Cancer in Palliative Care
“An observational study of patients with advanced cancer admitted to specialist palliative care units (SPCUs) found that 1 in 3 had deep vein thrombosis and that thromboprophylaxis may have little benefit for late-stage disease. These findings were recently published in The Lancet Haematology.
Prior to this study, the true prevalence of deep vein thrombosis in patients with advanced cancer was unknown. In addition, though thromboprophylaxis is recommended to prevent thromboembolism in the hospital setting, very few studies have determined whether thromboprophylaxis is effective specifically in patients with advanced cancer.
In this prospective, longitudinal, observational study, a cohort of 343 participants admitted to SPCUs were initially evaluated for several clinical characteristics, history of thromboembolism, and previous blood test results. The researchers then screened patients for deep vein thrombosis during disease progression.
The authors reported that the presence of deep vein thrombosis was predicted by
- a medical history of deep vein thrombosis (P =.011),
- having been previously bedbound (P =.003), and
- lower limb edema (P =.009).
Importantly, neither the use of thromboprophylactic agents, such as anticoagulants and antithromboembolism stockings, nor serum albumin levels were associated with the presence of deep vein thrombosis.
The results also indicated there was no difference in survival for patients with deep vein thrombosis (mean survival [MS] = 30.55 days, SD = 5.65) compared with patients without deep vein thrombosis (MS = 31.38 days, SD = 6.56; P =.432).
The authors concluded that their findings “[challenged] current recommendations for prevention of venous thromboembolism in advanced cancer” and raised additional questions about the timing and usage of thromboprophylaxis in this patient population.
Reference
1. White C, Noble SIR, Watson M, et al. Prevalence, symptom burden, and natural history of deep vein thrombosis in people with advanced cancer in specialist palliative care units (HIDDen): a prospective longitudinal observational study [published online February 1, 2019]. Lancet Haematol. doi: 10.1016/S2352-3026(18)30215-1