“Side effects (from CAR-T cell therapy) can be life-threatening, however. They include high fevers, crashing blood pressure, lung congestion and neurological problems.
No one wants CAR-T cell therapy to live up to its promise more than I do. I am a long-term survivor of an incurable blood cancer called multiple myeloma. MM is a blood cancer like the cancers discussed below. Like all chemotherapy development however, the challenge is to figure out the risk/reward proposition for you or your loved one.
Scroll down the page to learn more about multiple myeloma and Keytruda aka pembrolizumab.
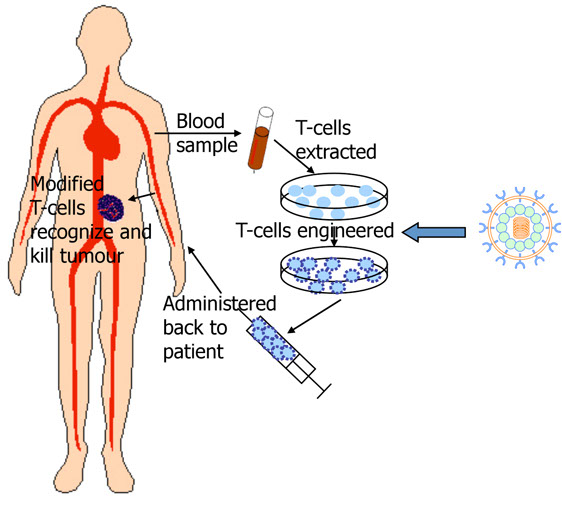
In the study for car-t cell approval discussed in the NYTimes article linked and excerpted below the in addition to serious life threatening collateral damage car-t cell therapy caused two deaths.
My conclusions of the current state of car-t cell chemotherapy development for MM:
- As of the writing of this post, Car-t cell therapy is not available for multiple myeloma survivors yet. Great to start with blood cancers but nothing for MM yet.
- Once Car-T cells are available for MM patients it is clear that the short, long-term and late stage side effects can be severe.
- Car-t cell therapy appears to be incredibly expensive. It is difficult to know just how expensive it will be for MMers at this point.
- For the first time ever a drug company is discussing the possibility of drug pricing based on performance…
My experience as a long-term MM survivor is that car-t cell development is relatively young. Most if not all chemotherapy development involves challenges like the ones outlined below. I plan to blog about the ongoing development of this potential chemo breakthrough in the future.
To Learn More about CAR-T Therapy and Myeloma- click now
Have you been diagnosed with multiple myeloma? What stage? What symptoms are you experiencing?
Thank you,
David Emerson
- Multiple Myeloma Survivor
- MM Cancer Coach
- Director PeopleBeatingCancer
Recommended Reading:
“The new therapy, Yescarta, made by Kite Pharma, was approved for adults with aggressive forms of a blood cancer, non-Hodgkin’s lymphoma, who have undergone two regimens of chemotherapy that failed…
About 3,500 people a year in the United States may be candidates for Yescarta. It is meant to be given once, infused into a vein, and must be manufactured individually for each patient. The cost will be $373,000…
Side effects can be life-threatening, however. They include high fevers, crashing blood pressure, lung congestion and neurological problems. In some cases, patients have required treatment in an intensive care unit. In the study that led to the approval, two patients died from side effects. Doctors have learned to manage them better, but it takes training and experience…
Novartis is expected to ask the F.D.A. to approve Kymriah for lymphoma and other blood cancers as well, and may vary its price depending on how well it works for those diseases…Kite also plans to seek approval for other blood cancers, but does not plan to vary Yescarta’s price, said Ms. Cassiano…
The company (Kite Pharma) also hopes that Yescarta will eventually be approved for earlier stages of lymphoma, rather than being limited to patients with advanced disease who have been debilitated by multiple types of chemotherapy that did not work…
The study that led to approval enrolled 111 patients at 22 hospitals; 101 of them received Yescarta. They had one of three diseases: diffuse large B-cell lymphoma, primary mediastinal B-cell lymphoma or transformed follicular lymphoma.
Initially, 54 percent had complete remissions, meaning that their tumors disappeared. Another 28 percent had partial remissions, in which tumors shrank or appeared less active on scans. After six months, 80 percent of the 101 were still alive.
With a median follow-up of 8.7 months, 39 percent of the 101 were still in complete remission — a much higher rate than achieved with earlier treatments — and 5 percent still had partial remissions…
Tina Bureau, a fifth-grade teacher from Queensbury, N.Y., was one of the lymphoma patients in the study. Previously, she’d had several types of chemotherapy…
Last spring, she had the T-cell treatment at the Dana-Farber Cancer Institute and Brigham and Women’s Hospital in Boston. The side effects were ferocious.
“You don’t even recognize your family members,” Ms. Bureau said. “I had some bleeding on my brain, and had to be put in intensive care. The week it was happening, I don’t remember a lot. It was much more difficult for my family than me.”
Within a month, she had a complete remission, which has continued. She is back at work, full time.
“Yes, it can pose life-threatening problems,” Ms. Bureau said. “But when you’re in a situation where your life’s threatened anyway, I don’t feel you have anything to lose.”
“Tisagenlecleucel (Kymriah, Novartis) was priced at $475,000, and axicabtagene ciloleucel (Yescarta, Kite Pharma) was priced at $373,000.
However, these are prices for the drug products alone. On top of this comes the cost of administering and managing a patient undergoing CAR T-cell treatment, which include leukapherisis, lymphodepletion therapy, and management of the adverse effects of CAR T.
Now, for the first time, there is an estimate of the total mean cost of the treatments in the United States: $510,963 for tisagenlecleucel, and $402,647 for axicabtagene ciloleucel…
The nondrug costs are $400,000 to $450,000, which is just about equal to the drug costs, he said. Thus, these additional costs double the total cost of the therapy.
“They are underestimating the non-T-cell costs by at least fivefold,” Silver told Medscape Medical News…
“The relatively new type of cancer treatment know as CAR T-cell therapy is already being used to treat some people with leukemia and lymphoma. This form of immunotherapy has also shown promise as a treatment for multiple myeloma, although it’s not yet approved by the Food and Drug Administration (FDA) for that use…”
PD-1/PD-L1 Inhibitors Pembrolizumab/Keytruda for Multiple Myeloma
“…the initially optimistic results (for PD-1/PD-L1 Inhibitors) have become disappointing due to the excessive and unpredictable toxicity of the combination of pembrolizumab with lenalidomide or pomalidomide.”
It doesn’t look good for PD-1/PD-L1 Inhibitors Pembrolizumab (Keytruda) according to the study linked and excerpted below. In fact, Keytruda faired worse than standard chemo regimens in just about every area of study-
- Progression-free survival-
- Overall survival-
- Adverse events-
To be fair, blood cancers in general and multiple myeloma specifically, is fundamentally different from solid tumor cancers. Further, the trials discussed below both used Keytruda with chemotherapy(lenalidomide or pomalidomide). That is a lot of toxicity.
Lastly, conventional oncology has had so much success with other chemotherapy regimens. I just wrote two blog posts about minimal residual disease in MM. One million bone marrow cells surrounding one myeloma cell.
Recommended Reading:
” Their (PD-1/PD-L1 Inhibitors) current application in multiple myeloma (MM) is rather uncertain, as discordant results have been reported by distinct research groups concerning especially the expression of PD-1/PD-L1 molecules on malignant plasma cells or on the responsible immune effector cell populations, respectively.
In MM it seems that an approach based on combination treatment might be appropriate as unsatisfactory results have been yielded by monotherapy with PD-1/PD-L1 inhibitors.
Immunomodulatory drugs, which are the current cornerstone of MM treatment, are the most logical partners as they possess many possibly synergistic effects. Nevertheless, the initially optimistic results have become disappointing due to the excessive and unpredictable toxicity of the combination of pembrolizumab with lenalidomide or pomalidomide.
The FDA has suspended or put on hold several phase 3 trials in relapsed as well as in newly diagnosed myeloma patients…”
No survival benefit with pembrolizumab in newly diagnosed or relapsed/refractory disease
“Adding the checkpoint inhibitor pembrolizumab (Keytruda) to standard therapy led to worse survival for patients with multiple myeloma, as compared with standard therapy with an immunomodulator and dexamethasone, two randomized trials showed.
Terminated early by the FDA, both trials had more deaths in the pembrolizumab-treated groups than in the control groups, almost twice as many in the KEYNOTE-185 study involving patients with newly diagnosed myeloma. Progression-free survival (PFS) also favored standard therapy in KEYNOTE 185 and in KEYNOTE 183, which included patients with relapsed/refractory myeloma. The pembrolizumab groups also had higher rates of toxicity…
Adding the checkpoint inhibitor pembrolizumab (Keytruda) to standard therapy led to worse survival for patients with multiple myeloma, as compared with standard therapy with an immunomodulator and dexamethasone, two randomized trials showed.
Although these data showed an imbalance in the number of deaths between treatment groups, the interim analyses were underpowered and inconclusive because of the shortened follow-up at termination. Additional studies are necessary to optimize identification of patients who would benefit from PD-1 inhibition in combination with pomalidomide (Pomalyst).”
The FDA halted enrollment in KEYNOTE 183 after accrual of 249 patients, determining that the risks of the three-drug combination outweighed the benefits. With a median follow-up of 8.1 months, the pembrolizumab group had a median PFS of 5.6 months versus 8.1 months with standard therapy.
The estimated 6-month PFS was 48% with pembrolizumab and 60% without. Median OS was not reached in either arm, but the pembrolizumab group had an increased survival hazard (HR 1.61, 95% CI 0.91-2.85). The estimated 6-month OS was 82% with pembrolizumab and 90% without it.
Serious adverse events (SAEs) occurred more often with pembrolizumab than with standard therapy alone (63% vs 46%). Four treatment-related deaths occurred in the pembrolizumab arm and none in the control group...