“The preliminary data from the two pilot studies suggested that AHCC supplementation supports the host immune system for successful clearance of HR-HPV infections.”
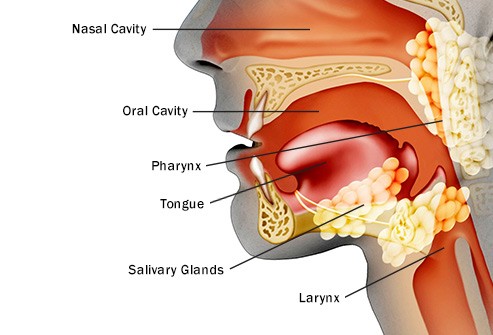
Hi David- I was diagnosed with throat cancer stage 3, in October ( tumor on base of tongue). Not from smoking but HPV positive in tumor. Have bilateral swollen lymph nodes. Largest being about 3.5 cm and other about 2.5cm. I have had swollen nodes for probably 5 to 6 years. i have had many sinus infections over the years and that is what brought me in to see ENT doctor. I feel better than I ever have due to My new nutritional ways. I have stopped all refined sugar, dairy and caffeine. Presently taking 12000 mg of lyposheric vit c every day.
I am always looking for additional things to do to defeat this! Thanks, Larry
Hi Larry-
If your stage and diagnosis are the type referred to in the study below. I am surprised that you are doing as well as you are. By this I mean that you say you have lived with squamous cell carcinoma of the tongue base for 5-6 years and are feeling well. The study below says that the 5 year survival rate of early stage SCCT (you five years ago) is only 9%.
In my experience, a combination of conventional, complementary and integrative therapies offers the cancer patient the best combination of length of life as well as quality of life.
For example, undergoing surgery to debunk the cancer is probably your best first step. Perhaps then undergoing local radiation to kill any local cancer that was not removed with surgery. I encourage hyperbaric oxygen therapy and acupuncture in order to prevent a common side effect of radiation called xerostomia.
Also consider AHCC supplementation which, according to the last study linked below, may clear your HPV virus.
Before then undergoing chemotherapy, consider evidence-based integrative therapies- therapies such as curcumin or whole-body hyperthermia shown to enhance the efficacy of your chemotherapy.
thanks and good luck,
David Emerson
- Cancer Survivor
- Cancer Coach
- Director PeopleBeatingCancer
Recommended Reading:
“Objectives: Squamous cell carcinoma of the tongue base has a poor prognosis, and treatment is accompanied by a number of major problems. In view of this, it is important to recognize which patients will benefit from treatment with curative intent and which treatment method to use…
Results: The 5-year cause-specific survival rate was 41% for those treated by irradiation, 58% for those treated by surgery, and 9% for untreated patients. There was no difference in the efficacy of treatment methods (p = .5362), but a highly significant difference was seen in survival rate between treated and untreated patients (p = .0028).
The decision regarding administration of curative treatment was based on the extent of locoregional involvement at the primary site (p = .0139; odds ratio, 0.43) and in the neck (p = .0078; odds ratio, 0.23). No factors affected the decision to treat by irradiation or surgery.
When the observed survival rate was calculated, there was no significant difference in 5-year survival rate between treated and untreated patients (p = .2762). Those with early (T1-2) disease at the primary site had an improved survival rate from 0.5 to 4 years compared with those who were untreated (T3-4; p = .0081; odds ratio, 2.2).
In addition, those with early (T1-2) disease had a better survival rate than those with advanced cancers (p = .0139; odds ratio, 2.09). There was, however, no difference in survival rate at 5 years. Those with early disease compared with those with advanced disease were twice as likely to be alive at 2 years; however, all survival advantages had disappeared by 5 years.
Conclusions: In terms of observed survival, treating tongue base squamous cell carcinoma that is locally advanced (T3-4) at presentation offers no survival advantage over palliation alone. Treating early disease (T1-2) doubles the survival rate for up to 4 years, but by 5 years this survival advantage is lost. The present study finds radiotherapy and surgery to be equivalent at controlling this disease.
“Head and neck squamous cell carcinoma (HNSCC) accounts for 6% of all malignancies in USA and unfortunately the recurrence of secondary primary tumors and resistance against conventional treatments decrease the overall 5 year survival rate in HNSCC patients…
GSE selectively inhibited the growth and caused cell cycle arrest and apoptotic death in both Detroit 562 and FaDu cells…
In terms of its toxicity and bioavailability, in vivo studies with GSE have shown that it is well tolerated and is considered safe as dietary supplement for human consumption…
In summary, the present study clearly demonstrated GSE efficacy against HNSCC cells both in cell culture and animal models…Considering the limited therapeutic options available against HNSCC, results from the present study support additional studies investigating GSE efficacy against HNSCC both in chemoprevention and intervention protocols and define the mechanisms of its efficacy.”
Objective: There is currently no effective medicine or supplement for clearance of high risk- human papillomavirus (HR-HPV) infections. We have taken a systematic approach evaluating the potential use of AHCC supplementation to support clearance of HR-HPV infections. The primary objective of this research was to evaluate AHCC supplementation to modulation of the host immune system to clear HR-HPV infections from bench to bedside.
Methods: Cervical cancer cells, CaSki (HPV16+), HeLa(HPV18+), SiHa(HPV16/18+), and C-33A(HPV–), were treated in vitrowith AHCC 0.42 mg/mL daily x7 days then observed x7 days with daily sample collection. A confirmatory study in cervical cancer mouse models, SiHa(HPV16/18+) and C-33A(HPV–), was conducted: mice were divided into three groups per cell line then dosed with AHCC 50 mg/kg/d (N= 10), or vehicle alone (N = 10), or no supplementation (N = 10) for a total of 90 days followed by 30 days of observation. Tumors were measured 3x/week and blood samples collected bi-weekly to evaluate interferon (IFN) alpha(α), beta(β), and gamma(γ) and immunoglobulin G(IgG) by immunoassays.
Tumors were evaluated for HR-HPV expression by PCR. Two pilot studies of 10 patients each were conducted in women with confirmed persistent HR-HPV+ infections. The 1ststudy evaluated AHCC 3g from 5 weeks up to 6 months and 2nd study evaluated AHCC 1g < 8 months. HR-HPV DNA status and the immune panel were monitored at each visit.
Results: HR–HPV clearance was observed in vitro and confirmed in the animal studies as a durable response. Four of six (66.7%) patients had confirmed HR-HPV clearance after 3-6 months of AHCC 3g. Similarly, 4 of 9 (44%) patients had confirmed HR-HPV clearance after 7 months of AHCC 1g. Suppression of IFNβ <25 pg/mL was observed in those clearing the HR-HPV infection.
Conclusion: Pre-clinical in vitro and in vivo studies demonstrated durable clearance of HR-HPV infections. The preliminary data from the two pilot studies suggested that AHCC supplementation supports the host immune system for successful clearance of HR-HPV infections. A confirmatory phase II randomized, double-blinded, placebo-controlled study is ongoing.