Recently Diagnosed or Relapsed? Stop Looking For a Miracle Cure, and Use Evidence-Based Therapies To Enhance Your Treatment and Prolong Your Remission
Multiple Myeloma an incurable disease, but I have spent the last 25 years in remission using a blend of conventional oncology and evidence-based nutrition, supplementation, and lifestyle therapies from peer-reviewed studies that your oncologist probably hasn't told you about.
Click the orange button to the right to learn more about what you can start doing today.
- You are here:
- Home »
- Blog »
- Multiple Myeloma »
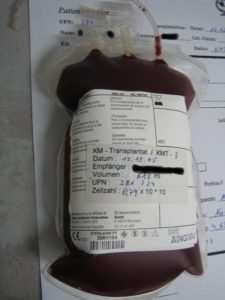
- Myeloma- Autologous Stem Cell Transplant-
Myeloma- Autologous Stem Cell Transplant-

The rate of grade 3 or 4 neutropenia was significantly higher in the transplantation group than in the RVD-alone group (92% vs. 47%), as were the rates of grade 3 or 4 gastrointestinal disorders (28% vs. 7%) and infections (20% vs. 9%).
You’ve been newly diagnosed with an incurable blood cancer called multiple myeloma. You’ve been told that the average life expectancy for MM patients is 5-7 years. You’ve heard lots of different things about how long people live with MM. You may have even heard people talk about “cures” or “beating the odds.” So what does this statistic of “5-7 years” mean?
Your oncologist is proposing you undergo the standard-of-care (SOC) therapy plan for MM. The SOC is the basic therapy plan researched and approved by the Food and Drug Administration.
The SOC for newly diagnosed multiple myeloma is:
- Induction Therapy (Revlimid, Velcade, Dexamethasone)
- An autologous stem cell transplant
- followed by maintenance therapy
According to the top study linked and excerpted below, the best case scenario for a long Progression Free Survival (PFS) is 10 plus years. 10 year for your PFS may then translate to a long overall survival (OS) of…maybe…20 years. But single digit chances of that.
The “long PFS” patients were:
- younger
- healthier (higher hemoglobin, higher platelet count, better kidney function)
- all low risk cytogenics
- early stage MM
If I read the study below correctly, if you are young, have early stage MM, have no genetic abnormalities and your MM is healthy as MM goes, you have a 9% chance of a function cure and then a good chance for a long overall survival. If your situation does not fit into this criteria, you then fall into the typical or average MM outcome.
- 9% of MM patients achieved a long PFS-
- 91% achieved a PFS of less than two years.
The problem with having an ASCT and achieving a long PFS, possibly a long OS, possibly a functional cure is the risk of serious side effects. According to the second and third studies linked below, the longer you live after standard-of-care, high dose, aggressive therapies including-
- RVD Induction therapy
- ASCT
- Maintenance therapy
the greater your risk of serious side effects. The studies below are not talking about nausea or loosing your hair. They are talking about
- endocrinopathies,
- musculoskeletal disorders,
- cardiopulmonary compromise and
- subsequent malignancies.
To summarize, if you are young, early MM, low risk, meaning you are in a GREAT situation to beat the odds and have a long PFS and possible a long OS, chances are you will develop serious side effects.
Consider low dose chemotherapy coupled with nutrition, anti-MM supplementation, and lifestyle therapies, ALL that have shown the ability to fight MM. No, these therapies are not the “standard-of-care” therapies for MM.
To Learn More about ASCT and Harvesting Stem Cells- click now
If you are curious to learn more, scroll down the page and comment. I will reply to you ASAP.
Thank you,
David Emerson
- MM Survivor
- MM Cancer Coach
- Director PeopleBeatingCancer
Recommended Reading:
- Multiple Myeloma in the Elderly- Minimize Toxicity-Increase Non-Toxic Therapies
- Multiple Myeloma Response – MRD, sCR, CR, VGPR, PR ???
- Relapsed/Refractory Multiple Myeloma- Patient Preferences
- How Long Can a Young Person Live with Multiple Myeloma?
Functional Cure, Defined As PFS of More Than 7 Years, Is Achieved in 9% of Myeloma Patients in the Era of Conventional Chemotherapy and of First-Generation Novel Anti-Myeloma Agents; A Single-Center Experience over 20-Year Period
“The aim of this analysis was to evaluate the characteristics of patients who achieved at least 7-year of PFS after frontline therapy and compare them with those of all other patients who were treated in a single center during the same time period (’94-’10)…
Between January 1994 and December 2010, 406 consecutive newly diagnosed MM patients received first line therapy in the Department of Clinical Therapeutics (Athens, Greece). All patients had symptomatic disease, based on the IMWG criteria of that period (at least one CRAB symptom to start anti-myeloma therapy).
These patients have:
- low risk disease,
- mainly of ISS-1 or -2,
- no high-risk cytogenetics,
- no or mild renal impairment,
- and achieve deep responses after ASCT.
The median OS of the whole group of patients was 5 years; in the long-PFS group median OS has not been reached yet while in all other patients the median OS was 4.3 years.
These patients may be considered as “functionally” cured. The incorporation of novel treatment approaches may lead to a significant improvement in the probability of achievement of this “functionally” cured status.
Lenalidomide, Bortezomib, and Dexamethasone with Transplantation for Myeloma.
Median progression-free survival was significantly longer in the group that underwent transplantation than in the group that received RVD alone (50 months vs. 36 months; adjusted hazard ratio for disease progression or death, 0.65; P<0.001).
This benefit was observed across all patient subgroups, including those stratified according to International Staging System stage and cytogenetic risk. The percentage of patients with a complete response was higher in the transplantation group than in the RVD-alone group (59% vs. 48%, P=0.03), as was the percentage of patients in whom minimal residual disease was not detected (79% vs. 65%, P<0.001).
Overall survival at 4 years did not differ significantly between the transplantation group and the RVD-alone group (81% and 82%, respectively).
The rate of grade 3 or 4 neutropenia was significantly higher in the transplantation group than in the RVD-alone group (92% vs. 47%), as were the rates of grade 3 or 4 gastrointestinal disorders (28% vs. 7%) and infections (20% vs. 9%).
No significant between-group differences were observed in the rates of treatment-related deaths, second primary cancers, thromboembolic events, and peripheral neuropathy.
CONCLUSIONS:
Among adults with multiple myeloma, RVD therapy plus transplantation was associated with significantly longer progression-free survival than RVD therapy alone, but overall survival did not differ significantly between the two approaches. (Supported by Celgene and others; IFM 2009 Study ClinicalTrials.gov number, NCT01191060 .).
Long-term health impacts of hematopoietic stem cell transplantation inform recommendations for follow-up
“… However, hematopoietic stem cell transplantation (HSCT) survivors are at risk of developing long-term complications, such as:
- endocrinopathies,
- musculoskeletal disorders,
- cardiopulmonary compromise and
- subsequent malignancies.
These complications have a direct impact on the morbidity and mortality experienced by HSCT survivors. Two-thirds of HSCT survivors develop at least one chronic health condition; while a fifth develop severe or life-threatening conditions.
HSCT patients who have survived for at least 5 years post-transplantation are at a fourfold to ninefold increased risk of late mortality for as long as 30 years from HSCT, producing an estimated 30% lower life expectancy compared with the general population.
The high burden of morbidity experienced by HSCT survivors makes it critically important that there is standardized follow-up of HSCT survivors at high risk for post-HSCT complications. The Center for International Blood and Marrow Transplant Research/European Group for Blood and Marrow Transplantation/American Society for Blood and Marrow Transplantation and the Children’s Oncology Group long-term follow-up guidelines offer such standardized care. Future steps include wider dissemination and refinement of these guidelines…”