It’s manufactured by Bayer and marketed by Janssen, a Johnson & Johnson company, and one of their best-selling drugs — namely as doctors recommend more aging Baby Boomers regularly take blood thinners…
Xarelto was sold on safety and convenience, as it required fewer tests and was less likely to have adverse reactions with other drugs and food, according to Harvard Health.
Its main advantage was that tests suggested it was safer than the generic warfarin, namely there was a lowered risk of stroke or uncontrollable bleeding.
But safety concerns are the center of a large settlement regarding tens of thousands of lawsuits that claimed Bayer and Johnson & Johnson didn’t adequately warn patients of a serious side effect: bleeding.
On March 25th, Bayer and Johnson & Johnson announced they would be splitting a $775 million settlement to quash 25,000 claims that the companies didn’t adequately warn patients of the increased bleeding risk. That’s $31,000 per claim, which patients will only see a fraction of after attorney fees.
In their announcements, Bayer and Johnson & Johnson were quick to note they had prevailed when six of those claims went to trial.
“We found that the label clearly communicated Xarelto’s benefits and risks, and that claims plaintiffs made about the medicine did not square with the facts,” Bayer’s statement said, calling the allegations “meritless.”
The statement further explained they settled because news coverage of the lawsuits and ads from law firms “can create barriers to the critical doctor-patient relationship and complicate decisions about treatment options.”
Both companies stand by Xarelto, noting the 45 million patients worldwide who have been prescribed the medication in the last eight years. As part of the settlement, neither company admitted liability.
Caitlin Hoff, a consumer advocate with ConsumerSafety.org — a website run by lawyers involved in the Xarelto lawsuits — says the litigation highlights the public’s desire “for greater scrutiny and transparency in the drug approval process.”
“These lawsuits specifically recognize the thousands of patients who were misinformed or simply were not fully aware of the risks that they were taking and suffered the consequences,” Hoff told Healthline. “The lack of transparency left these patients without key information necessary to make safe medical decisions for themselves and their families.”
Since then, as concerns were raised and lawsuits were filed, the U.S. Food and Drug Administration (FDA) continued to approve Xarelto for even more conditions.
Xarelto’s problems began with a device used to test bleeding risk before it was approved by the FDA in 2011.
As Healthline has previously reported, Xarelto’s approval process involved:
- using a device that tested bleeding — one the FDA later recalled
- warning letters from the FDA to both that device maker and to Xarelto’s parent companies for understating bleeding risks
- safety studies conducted mainly by researchers paid by either Bayer, Janssen, or Johnson & Johnson
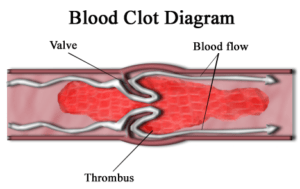
One other aspect of the thousands of lawsuits surrounding Xarelto was the lack of an antidote for the bleeding problem associated with most new blood thinners.
The FDA approved an antidote called andexanet alfa for Xarelto and other newer blood thinners in May 2018.
Given all this information, people who are on Xarelto, or those caring for those who are, may have some concerns. Experts say it’s best to take those concerns straight to the doctor or specialist who prescribed the blood thinner…
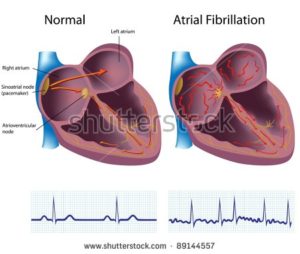
“Ultimately, patients need to decide with their doctor if the bleeding risk of anticoagulants like Xarelto is worth accepting, in order to prevent strokes associated with atrial fibrillation or other clotting problems,” he said…
Even with years of lawsuits filed against it, Xarelto’s market share continues to rise, even as similar drugs from competing manufacturers receive FDA approval…
Consumer advocate Hoff says she doubts the settlement will affect much of the FDA’s drug approval process or encourage drug manufacturers to be more transparent in the future, but the attention to the lawsuit and settlement “may build awareness in more Americans and push consumers to fight for change in the FDA’s regulatory process.”
“This settlement is another example of a large pharmaceutical company paying a relatively small fee for their blatant negligence,” Hoff said. “They will continue to sell and profit off of Xarelto and its antidote, and without greater retribution, there is little motivation for them to change their business practices.”