
Recently Diagnosed or Relapsed? Stop Looking For a Miracle Cure, and Use Evidence-Based Therapies To Enhance Your Treatment and Prolong Your Remission
Multiple Myeloma an incurable disease, but I have spent the last 25 years in remission using a blend of conventional oncology and evidence-based nutrition, supplementation, and lifestyle therapies from peer-reviewed studies that your oncologist probably hasn't told you about.
Click the orange button to the right to learn more about what you can start doing today.
- You are here:
- Home »
- Blog »
- Multiple Myeloma »
- Myeloma- Stem Cell Transplant
Myeloma- Stem Cell Transplant
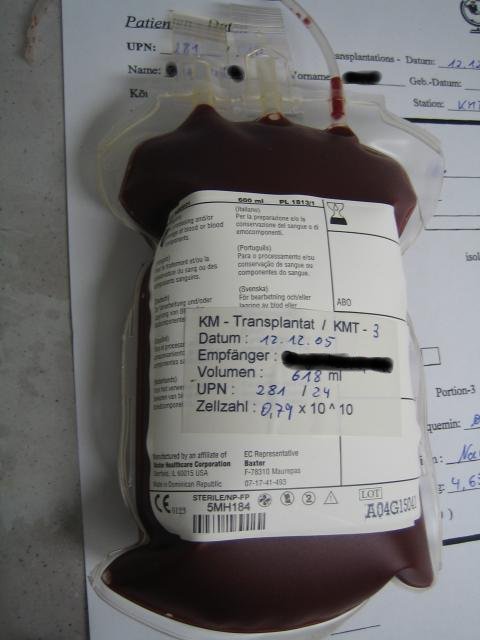
Why do myeloma patients undergo a stem cell transplant? Will you live longer by undergoing aggressive, high dose chemotherapy? The study linked below says no.
“Finally, in 1995, the first randomized trial was published, with impressive results…But these results didn’t hold up…The approach was recognized for what it was: ineffective at best, lethal at worst…”
The NYT article linked and excerpted below is a great read discussing the concept of “evidence-based medicine.” Being a multiple myeloma (MM) blogger I have chosen to focus this post on the article’s discussion of one medical procedure that the article calls “ineffective at best, lethal at worst.” Standard-of-care multiple myeloma treatment- an autologous stem cell transplant (ASCT).
To be fair, when the article below talks about an autologous stem cell transplant, it is referring to the history of the procedure for breast cancer, not multiple myeloma. My perspective however, is that of a long-term MM survivor who’s ASCT in 1995 did not work. My ASCT put me in partial remission for less than 10 months and I spent the next 25 years struggling with the short, long-term and late stage side effects brought about by aggressive, high dose chemotherapy. Which is what an ASCT is.
The issues that jump out at me in the article below are two-
- experimental treatments
- invested stakeholders
Could two phrases from the article below be more different when writing a blog post about multiple myeloma?
Hear me out. I remember the conversation with Dr. Lazarus like it was yesterday. Dr. Lazarus explained to me that an autologous stem cell transplant for multiple myeloma treatment was considered to be “experimental” and therefore would not be covered by my health insurance. Dr. Lazarus assured me however, that he would write to Medical Mutual of Ohio, my insurer, and get them to cover the procedure.
And he was correct. Medical Mutual of Ohio did pay for my ASCT. Which, as I said, was ineffective. So when I was told that there was “nothing more they (UH) could do for me” in 9/97, I underwent another “experimental” therapy. Antineoplaston Therapy from the Burzynski Research Institute. I went from end-stage MM to complete remission in less than 17 months.
Unfortunately, MMO wouldn’t pay for my experimental therapy this time. Fortunately, I reached complete remission where I have remained until today, 8/19.
To this day (8/30/19) numerous studies for ASCT for multiple myeloma have never shown an overall survival (length of life) benefit. Studies have shown only that ASCT for MM patients can provide, on average, a longer first remission (progression-free survival).
Two very different experimental treatments, two very different outcomes. The experimental procedure that was paid for has “invested stakeholders” in the form of doctors, hospitals, and drug companies and is now consider “standard-of-care” for multiple myeloma patients. The experimental procedure that was not paid for, is considered to be controversial (quack medicine) and has only one invested stakeholder Stanislaw Burzynski, MD, Phd.
I believe the term “evidence-based” in conventional multiple myeloma treatment, is meaningless.
To Learn More about ASCT and Harvesting Stem Cells- click now
Thank you.
David Emerson
- MM Survivor
- MM Cancer Coach
- Director PeopleBeatingCancer
Recommended Reading:
- Cannabinoids, C-B-D for Myeloma Pain Management
- Your Diagnosis of Multiple Myeloma- A How-To Guide
- Autologous Stem Cell Transplant for Multiple Myeloma- What you Need to Know
Evidence-based medicine has made progress since doctors’ infamous bloodletting of George Washington, but less than you might think.
The British Medical Journal sifted through the evidence for thousands of medical treatments to assess which are beneficial and which aren’t. According to the analysis, there is evidence of some benefit for just over 40 percent of them. Only 3 percent are ineffective or harmful; a further 6 percent are unlikely to be helpful. But a whopping 50 percent are of unknown effectiveness. We haven’t done the studies.
Sometimes uncertain and experimental treatments are warranted; patients may even welcome them. When there is no known cure for a fatal or severely debilitating health condition, trying something uncertain — as evidence is gathered — is a reasonable approach, provided the patient is informed and consents.
“We have lots of effective treatments, many of which were originally experimental,” said Dr. Jason H. Wasfy, an assistant professor of medicine at Harvard Medical School and a cardiologist at Massachusetts General Hospital. “But not every experimental treatment ends up effective, and many aren’t better than existing alternatives. It’s important to collect and analyze the evidence so we can stop doing things that don’t work to minimize patient harm.”


