Diagnosed with Cancer? Your two greatest challenges are understanding cancer and understanding possible side effects from chemo and radiation. Knowledge is Power!
Learn about conventional, complementary, and integrative therapies.
Dealing with treatment side effects? Learn about evidence-based therapies to alleviate your symptoms.
Click the orange button to the right to learn more.
- You are here:
- Home »
- Blog »
- side effects ID and prevention »
- Treatment-Related Secondary Cancer
Treatment-Related Secondary Cancer

Treatment-related secondary cancer is a little discussed but every present side effect of just about every conventional cancer therapy.
When I read the article linked below I was inspired to post about the quote
“Patients and clinical trial participants receiving treatment with these products should be monitored lifelong for new malignancies,” Dr Marks said.
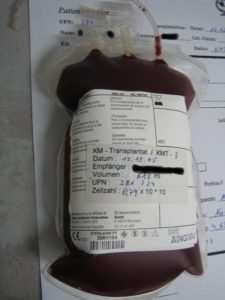
I had an autologous stem cell transplant (ASCT) in late 1995. An ASCT is the technical name for aggressive, high dose chemotherapy. This was proceeded by lots of chemotherapy and radiation. My point is that everyone who gets this much tumor inducing therapy should be monitored for life!
The serious risk of short, long-term and late stage side effects such as treatment-related secondary cancer is huge.
Several cancer therapies can increase the risk of secondary cancers, also known as second primary cancers. These therapies include:
- Radiation Therapy: While radiation therapy is used to kill cancer cells, it can also damage healthy cells in the treatment area. This damage may lead to the development of secondary cancers years after the radiation treatment. The risk depends on various factors such as the dose and location of radiation.
- Chemotherapy: Some chemotherapy drugs are known to increase the risk of secondary cancers. Certain chemotherapy agents, especially alkylating agents and topoisomerase II inhibitors, can cause DNA damage in healthy cells, potentially leading to the development of secondary cancers.
- Immunotherapy: While immunotherapy has revolutionized cancer treatment by enhancing the immune system’s ability to fight cancer, it can also lead to immune-related adverse events, including the development of secondary cancers. However, the risk appears to be lower compared to traditional chemotherapy and radiation therapy.
- Targeted Therapy: Targeted therapy drugs are designed to interfere with specific molecules involved in cancer cell growth and survival. Although these drugs are often more selective than traditional chemotherapy, they can still have off-target effects that may increase the risk of secondary cancers.
- Hormone Therapy: Hormone therapy is commonly used to treat hormone-sensitive cancers such as breast and prostate cancer. However, long-term hormone therapy may increase the risk of secondary cancers, particularly in the breast, uterus, or other hormone-sensitive organs.
- Autologous stem cell transplant-
Have y9u had an ASCT? Have you had CAR-T therapy? If you’d like to learn more about reducing your risk of treatment-related secondary cancer send me an email David.PeopleBeatingCancer@gmail.com
thank you,
David Emerson
- MM Survivor
- MM Cancer Coach
- Director PeopleBeatingCancer
CAR T Cell: Do Benefits Still Outweigh Risks?
“Reports of a small number of patients developing secondary T-cell malignancies following treatment with chimeric antigen receptor (CAR) T-cell immunotherapy have raised concerns and prompted a class-wide boxed warning to the labeling of the therapies by the US Food and Drug Administration (FDA), but for now experts underscore that the benefits of the groundbreaking therapies still appear to well outweigh the risks.
Importantly, most specialists agree, so far the risk appears no greater than the known risk of secondary primary malignancies that is well established with other cancer therapies.
“The data that we have so far suggest that the risk of secondary T-cell lymphoma in patients treated with CAR T-cells is similar to [that] of patients treated with other cancer therapies, [including] chemotherapy, radiation, transplantation,” Marco Ruella, MD, said in an interview. He reported on a case of a T-cell lymphoma occurring following CAR-T therapy at the University of Pennsylvania.
While his team is still investigating the development of such malignancies, “the FDA notice does not change our clinical practice and patients should be reassured that the benefit of CAR-T therapy significantly outweighs the potential risk of secondary malignancies including T-cell lymphoma,” said Dr Ruella, scientific director of the Lymphoma Program, Division of Hematology and Oncology and Center for Cellular Immunotherapies, at the University of Pennsylvania, Philadelphia.
FDA: 28 Reports of Malignancies; 3 With Evidence of ‘Likely’ CAR T Involvement
Concerns were raised last November when the FDA announced in a safety communication that it was investigating the “serious risk of T-cell malignancy” following B-cell maturation antigen (BCMA)-directed or CD19-directed CAR T-cell immunotherapies, citing reports from clinical trials and/or postmarketing adverse event data sources. Subsequently, in January, the FDA called for the boxed warning on all approved BCMA- and CD19-targeted genetically modified autologous T-cell immunotherapies, which include: Abecma (idecabtagene vicleucel); Breyanzi (lisocabtagene maraleucel); Carvykti (ciltacabtagene autoleucel); Kymriah (tisagenlecleucel); Tecartus (brexucabtagene autoleucel); and Yescarta (axicabtagene ciloleucel).
“Although the overall benefits of these products continue to outweigh their potential risks for their approved uses, the FDA continues to investigate the identified risk of T-cell malignancy with serious outcomes, including hospitalization and death,” the FDA reported in discussing the safety warnings.
The cases were detailed in a report from FDA researchers published in the New England Journal of Medicine, noting that as of December 31, 2023, the FDA had become aware of 22 cases of T-cell cancers occurring following CAR T-cell treatment, including T-cell lymphoma, T-cell large granular lymphocytosis, peripheral T-cell lymphoma, and cutaneous T-cell lymphoma.
Report coauthor Peter Marks, MD, PhD, of the FDA’s Center for Biologics Evaluation and Research in Silver Spring, Maryland, said in an interview that since the publication of their report, six new cases have emerged.
“As reported in the NEJM Perspective, there were 22 cases of T-cell malignancy after treatment with CAR T-cell immunotherapies as of December 31, 2023, but we have received additional reports and, as of February 9, 2024, FDA has now received 28 reports,” he said. “Note that as new cases are being reported, there will be updates to the total number of cases under ongoing review by FDA.”
The initial 22 cases all occurred relatively soon after treatment. Of 14 cases with sufficient data, all developed within 2 years of the CAR-T therapy, ranging from 1 to 19 months, with about half occurring in the first year after administration.
The cases involved five of the six FDA-approved CAR-T products, with the numbers too low to suggest an association with any particular product.
In three of the cases, the lymphoma was found in genetic testing to contain the CAR construction, “indicating that the CAR-T product was most likely involved in the development of the T-cell cancer,” according to the FDA researchers.
With inadequate genetic sampling in most of the remaining 19 cases, the association is less clear, however “the timing of several of the cases makes association a possibility,” Dr Marks said. In their report, Dr Marks and colleagues added that “determination of whether the T-cell cancer is associated with the CAR construct…most likely won’t be possible for every case reported to date.”
Even if all the reported cases are assumed to be related to CAR-T treatment, the numbers still represent a very small proportion of the more than 27,000 doses of the six CAR-T therapies approved in the United States, the authors noted, but they cautioned that the numbers could indeed be higher than reported.
“Relying on postmarketing reporting may lead to underestimates of such cases,” they said.
Life-Long Monitoring Recommended
In response to the reports, the FDA is urging that clinicians’ monitoring of patients treated with CAR-T therapy should be lifelong.
“Patients and clinical trial participants receiving treatment with these products should be monitored lifelong for new malignancies,” Dr Marks said.
“In the event that a new malignancy occurs following treatment with these products, contact the manufacturer to report the event and obtain instructions on collection of patient samples for testing for the presence of the CAR transgene.”