Recently Diagnosed or Relapsed? Stop Looking For a Miracle Cure, and Use Evidence-Based Therapies To Enhance Your Treatment and Prolong Your Remission
Multiple Myeloma an incurable disease, but I have spent the last 25 years in remission using a blend of conventional oncology and evidence-based nutrition, supplementation, and lifestyle therapies from peer-reviewed studies that your oncologist probably hasn't told you about.
Click the orange button to the right to learn more about what you can start doing today.
- You are here:
- Home »
- Blog »
- Multiple Myeloma »
- Celgene Spanked-Fixing Multiple Myeloma Drugs Thalidomide, Revlimid
Celgene Spanked-Fixing Multiple Myeloma Drugs Thalidomide, Revlimid
“Celgene charges up to $500 per capsule for the drugs (Thalidomide, Revlimid), and has taken in $17.1 billion selling them (to MM patients and survivors) from 2009 to 2013, the suit claimed.”
Considering Celgene has made over 17 billion (B) from the sale of Thalidomide and Revlimid to multiple myeloma (MM) patients and survivors, a fine of 55 million for any wrongdoing is a pittance.
It’s essential for MM patients and survivors to remember that cancer is a business. In the case of selling MM therapies, it is a lucrative business.
Having said that, I need to go on record as saying that I don’t believe drug companies such as Celgene are evil. I believe that creating, testing, selling drugs should be a for-profit business.
As a business person myself in my pre-MM life, I believe that capitalism can work well. I’m simply writing this blog post to draw attention to Celgene not playing by the rules.
Generic drug-“A generic drug is a pharmaceutical drug that contains the same chemical substance as a drug that was originally protected by patents…”
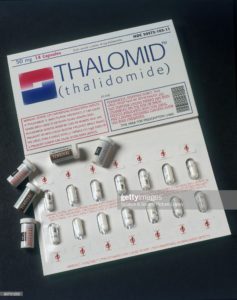
Thalidomide-“Thalidomide, sold under the brand name Thalomid and Inmunoprin, among others, is an immunomodulatory drug and the prototype of the thalidomide class of drugs.”
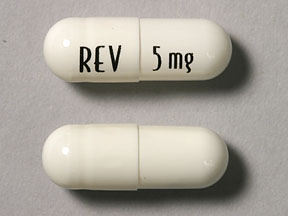
Lenalidomide-“Lenalidomide (trade name Revlimid) is a derivative of thalidomide approved in the United States in 2005….It costs $163,381 per year for the average patient.[3]
Have you been diagnosed with multiple myeloma? To learn more about evidence-based therapies shown to enhance the efficacy of both Thalidomide and Revlimid, scroll down the page, post a quetion or comment and I will reply to you ASAP.
Thank you,
David Emerson
- MM Survivor
- MM Cancer Coach
- Director PeopleBeatingCancer
Recommended Reading:
- Nutrition as Cancer Therapy Before, During After Therapy
- Gut Health Leads Multiple Myeloma to MRD- Status
- Second Opinion from a Long-Term Multiple Myeloma Survivor, Cancer Coach
Celgene to Pay $55M to Settle Claims It Fixed Prices of Cancer Drugs
The drugmaker was accused of antitrust violations for allegedly interfering with development of generic versions of two of its drugs.
“Drugmaker Celgene has agreed to pay $55 million to settle a class action lawsuit accusing it of violating antitrust laws by interfering with the development of generic versions of two of its cancer drugs.
Plaintiffs in the class action, a group of labor unions and other end payers, moved Wednesday for preliminary approval of the settlement. The suit claimed Celgene repeatedly hiked the prices of its drugs Thalomid and Revlimid while pursuing an anti-competitive scheme against at least 11 makers of generic drugs…
Celgene charges up to $500 per capsule for the drugs, and has taken in $17.1 billion selling them from 2009 to 2013, the suit claimed…
Thalomid is also known as thalidomide, which was developed in the 1950s as a treatment for morning sickness in pregnant women, but it was taken off the U.S. market in 1962 after it was found to cause severe birth defects.
It was approved in 1998 as a treatment for leprosy, and more recently was used in conjunction with steroids to treat multiple myeloma.
Revlimid is similar to Thalomid and is also used for myeloma. Approval in 1998 was contingent on participation in the REMS program to ensure pregnant women do not use the drugs.
The Thalomid and Revlimid litigation claimed Celgene violated the Clayton Act and Sherman Act, and raised various antitrust, unfair trade practices and unjust enrichment claims under the laws of several states…
Generic equivalents of Thalomid and Revlimid were delayed for years due to the conduct of Celgene, the suit claimed.
The settlement class consists of people or entitles who paid the purchase price of Thalomid or Revlimid in California, the District of Columbia, Florida, Kansas, Maine, Massachusetts, Michigan, Nebraska, New York, North Carolina, Oregon, Pennsylvania, Rhode Island or Tennessee, for consumption by themselves, their families, or their members, employees, insureds, participants or beneficiaries.
Whitney Street, a Block & Leviton partner who worked on the case, said in a statement, “We are pleased to have obtained a substantial recovery on behalf of cancer patients and other parties that bear the skyrocketing cost of these lifesaving drugs.””