“They found that 14 drug trial reports did not include data on SAE s, 22 presented no data on serious events, and two presented no data on deaths (FAE)“
Don’t expect your oncologist to warn you that multiple myeloma chemotherapy he/she is giving you is toxic and may kill you. He/she may or may not know about the chemotherapy trialed and its toxic effects.
Clinical trial data, according to the study linked and excerpted below, downplays or even omits critical information about chemotherapy treatment-related serious adverse-events (SAE) and/or fatal adverse events (FAE).

Doctor using digital tablet with medical icon and heartbeat rate in the hospital background
Keep in mind that clinical trials, if the data is published, as a rule, put chemotherapy regimens in the most positive light possible. Further, keep in mind that clinical trials have inclusion and exclusion data insuring that those in the clinical trial are the healthiest patients possible. Lastly, clinical trials are for a set period of time. Long-term and late stage side effects are often not tracked.
Which is why the article below is so personal to me. I was diagnosed with multiple myeloma (MM) in early 1994. Once my conventional therapies ended and I had “failed” many different chemotherapy regimens, my oncologist told me that “we can do nothing more for you,” I had to move on. If you had seen me in September of 1997, you would have thought I was a perfectly healthy 37 year old man.
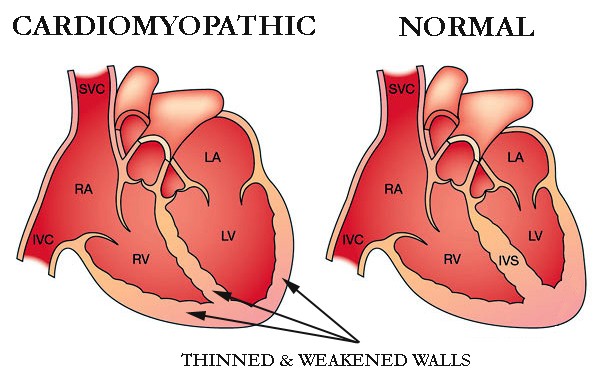
It was the next 25 plus years that led to heart, nerve and brain damage aka late-stage and long term side effects. If you’re thinking that it was the toxicity of chemotherapy and radiation that allowed me to live another 25 plus years, you would be wrong. Before my oncologist told me that nothing more could be done for me, Dr. Rassiga told me that I was end-stage.
It was an alternative therapy that took me from end-stage to complete remission in about 17 months.
Which brings me to the most important issue in the article below. When Dr. Weber is quoted as saying
“When you compare the [cancer drug trial] outcome for patients to death from cancer, then it becomes a little clearer what the term ‘acceptable’ or ‘tolerable’ means,” Weber said.
Giving newly diagnosed cancer patients a choice between:
- toxic chemotherapy that can cause SAE/FAE s and
- death by cancer
is a false equivalence.
Newly diagnosed multiple myeloma patients have no way of understanding SAE/FAE and newly diagnosed MM patients may or may not die.
What are the most common adverse events for myeloma chemotherapy?
- Bone marrow suppression: Chemotherapy drugs can suppress the bone marrow’s ability to produce blood cells, leading to decreased levels of white blood cells, red blood cells, and platelets. This can increase the risk of infections, anemia, and bleeding.
- Nausea and vomiting: Many chemotherapy drugs can cause nausea and vomiting, which can range from mild to severe. Anti-nausea medications are often prescribed to help manage these symptoms.
- Fatigue: Chemotherapy can cause fatigue, which may persist throughout treatment and even after treatment ends.
- Hair loss: Some chemotherapy drugs can cause hair loss, although not all drugs have this side effect.
- Peripheral neuropathy: Certain chemotherapy drugs can damage the nerves in the hands and feet, leading to symptoms such as numbness, tingling, or pain.
- Digestive issues: Chemotherapy can cause digestive problems such as diarrhea, constipation, or loss of appetite.
- Mucositis: Chemotherapy can irritate the mucous membranes lining the mouth and digestive tract, leading to mouth sores or difficulty swallowing.
- Increased risk of infection: Due to bone marrow suppression, chemotherapy can increase the risk of developing infections. Patients may need to take precautions to avoid exposure to infectious agents.
- Skin changes: Some chemotherapy drugs can cause changes to the skin, such as rashes, dryness, or increased sensitivity to sunlight.
- Cardiotoxicity: Certain chemotherapy drugs can have adverse effects on the heart, leading to conditions such as cardiomyopathy or arrhythmias.
I was told that my cancer, multiple myeloma, was incurable. As a newly diagnosed MM patient I had no way of understanding the late stage and long-term side effects that awaited me. And the kicker is that none of the late-stage and long term side effects had to happen. They all could have been minimized or prevented with evidence-based non-toxic therapies.
Have you been diagnosed with multiple myeloma? Have you been told that your cancer is incurable? Scroll down the page, post a question or comment and I will reply to you ASAP.
Thanks
David Emerson
- MM Survivor
- MM Cancer Coach
- Director PeopleBeatingCancer
Recommended Reading:
“A review of more than 100 randomized drug trials published in five top-tier clinical journals shows that 43% used words such as “tolerable,” “favorable,” “acceptable,” “manageable,” “feasible,” and “safe” to describe adverse events.
This language downplays treatment-related harms, which often appeared among data in tables but not in the narrative of the report, say the authors.
Some studies did not include data on serious adverse events (SAEs) or fatal adverse events (FAEs)…
The report was published online November 1 in the BMJ.
“We consider the lack of harms reporting and the use of subjective terms to describe harms to be poor reporting practice,” say the authors…
“Not fully reporting the harms of cancer drugs is of particular concern because cancer drugs usually provide modest benefits at high costs &mdash in terms of both price and toxicities,” they comment. “Downplaying harms can suggest a better risk-benefit profile than actually exists.”
Had the authors talked to patients, “they would have been surprised to learn the degree to which patients are able and willing to endure side effects for the chance of gaining benefit from treatment,”
“When you compare the [cancer drug trial] outcome for patients to death from cancer, then it becomes a little clearer what the term ‘acceptable’ or ‘tolerable’ means,” he said.
Patients will endure significant side effects in return for modest benefits. Dr Jeffrey Weber,“In my experience of 30 years as an investigational oncologist, patients will endure significant side effects in return for modest benefits,” Weber told Medscape Medical News.
Downplaying Harms
The authors of the review assessed 122 cancer drug trials published in 2016 in the
- New England Journal of Medicine,
- Lancet,
- Lancet Oncology,
- the Journal of the American Medical Association, and
- the Journal of Clinical Oncology.
They found that 14 drug trial reports did not include data on SAEs, 22 presented no data on serious events, and two presented no data on deaths.
In the 39 trials that did report harms data, the investigators found that
- the rates of SAE s were higher in the experimental arm than in the control arm in 30 trials (77%),
- SAE s were higher in 26 of 31 trials (84%), and
- deaths were higher in 34 of 51 trials (66%).
“Thus, despite using terms such as ‘favorable’ and ‘tolerable’ to describe the harms profiles of new treatments, the trials often showed a greater number of harms than in the control arms,” the authors say…
“How can anyone publish a trial report of a drug, claim acceptable toxicities, not report data on FAE, and claim that toxicities data are confidential? That was the biggest surprise of all,” he said.
Before the study began, Gyawali said the reviewers were familiar with the terms used to downplay toxicities associated with drug therapy, having heard them used at conferences and having read them in abstracts. They assumed this kind of language, which is discouraged in the CONSORT guidelines for reporting harms, would not survive the editorial process at leading clinical journals.
Instead, they found that in an alarming number of cases, benefits information, even with surrogate endpoints such as improved responses, was overly emphasized, whereas harms data, such as the number of drug-related deaths, were downplayed or not reported…
“The difference in treatment related serious adverse events-leading to
- death,
- life threatening condition,
- hospital admission or prolonged admission,
- disability or
- permanent damage,
- congenital anomaly or
- birth defect, or that
- required medical or surgical intervention to prevent one of the other outcomes)
was nearly five times higher,” they write…
Weber also noted that in most clinical drug trials, toxicities are reported for fixed periods during treatment. When patients remain on drug therapy for longer periods or finish therapy and experience late side effects, these may not be reported…