“…new treatment formulation delivered through a skin patch, maintaining an optimal blood level of the medicine (revlimid) for prolonged periods of time.”
Low-dose multiple myeloma maintenance therapy has been shown, in some studies, to improve overall survival of MM patients and survivors. Some not. Revlimid pill or Revlimid skin patch?
Revlimid (lenalidomide) is the standard-of-care multiple myeloma (MM) chemo regimen used most often for low-dose maintenance therapy.
According to ASCO, a 10mg pill of revlimid is taken in 28 day cycles until disease progression or until unacceptable toxicity.
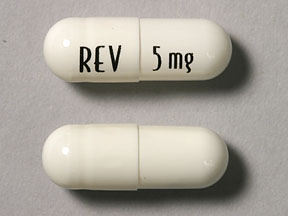
While in some studies, low-dose revlimid MM maintenance therapy has been shown to increase both progression-free survival (PFS) as well as overall survival (OS) for myeloma patients, MMers can develop side effects as well as chemoresistance to revlimid at doses of 10-25 mg a day.
What if low-dose MM maintenance therapy became was an even LOWER dose and the dosing regimen didn’t allow a person’s myeloma to have a therapy vacation EVER.
Consistent dosing throughout the 24 hour cycle should mean that the MM wouldn’t have a chance to develop resistance. An oral dose (pill) of revlimid does start strong during the 24 hour period an taper off as the hours pass.
What if the lenalidomide patch (revlimid skin patch), discussed below, was, say, the equivalent of a 5 mg a day dose, 24/7? Think nicotine patch for cancer patients. What if the average MM experienced less toxicity/lower daily dose, and had a consistent daily dose? Could MM maintenance therapy consist of a low-dose revlimid patch for years? For the rest of their lives?
While I doing the “what if” thing, what if myeloma patients were also undergoing evidence-based therapies shown to both kill their myeloma as well as enhance the efficacy of revlimid?
I admit that there are a lot of “what ifs.” Just an idea.
Thanks,
David Emerson
- MM Survivor
- MM Cancer Coach
- Director PeopleBeatingCancer
Recommended Reading:
“In summary, lenalidomide maintenance therapy significantly improved progression-free survival in patients with newly diagnosed multiple myeloma compared with observation, but overall survival was not improved in the intention-to-treat analysis across the whole trial population.
Additionally, prespecified subgroups analyses by cytogenetic risk and transplantation status suggested a progression-free survival benefit across all cytogenetic risk groups, and an overall survival benefit in transplantation-eligible patients…”
“Toxic effects and chemoresistance are major hurdles in chemotherapy and to avoid these problems caused by traditional chemotherapeutic regimens, a new modality of drug administration called “metronomic chemotherapy” has emerged. Such regimen involves the frequent administration of conventional chemotherapeutic agents at very low doses to target activated endothelial cells in tumors, the advantages of which include minimal adverse effects and a rare chance of developing acquired drug resistance…”
“Aiming to improve lenalidomide‘s safety and efficacy, ChemioCare is setting up to develop a new treatment formulation delivered through a skin patch, maintaining an optimal blood level of the medicine for prolonged periods of time.
The new formulation uses the company’s permeation-enhanced transdermal technology (PETT), which “works like a continuous injection of drug into the bloodstream that can be precisely delivered to provide the right amount of drug to work without providing too much drug, which can cause toxicity,” Jamie Oliver, ChemioCare’s chief marketing officer, said in a press release.
Lenalidomide — marketed as Revlimid by Celgene — is the standard of care for multiple myeloma patients, and is approved for some patients with myelodysplastic syndrome and mantle cell lymphoma.
The treatment is an immunomodulatory agent that stimulates the immune system to fight cancer and blocks the formation of new blood vessels around the tumor, preventing tumor growth through these two mechanisms.
But despite being delivered orally, which is more convenient for patients than intravenous infusions, Revlimid has significant dose-dependent side effects, such as low numbers of white blood cells, which ultimately reduces its tolerance and leads to dose reductions or treatment discontinuation.
The biggest problem with Revlimid, researchers say, are its fluctuating levels. The agent reaches its highest concentration after the pill is taken, but levels then drop continuously until the next dose. While the highest dose may cause treatment toxicity, the lowest levels may lead to treatment failure.
“Oral medications just are not able to precisely maintain the optimal blood levels over the dosing interval,” Oliver said.
Thus, a possible solution is to keep lenalidomide at its optimal levels at all times, which might be achieved via a device that continuously releases the agent into circulation…
Because a lenalidomide formulation is already approved, this skin patch leverages the 505(b)2 regulatory path, allowing it to enter Phase 3 trials directly without the need for prior safety, dose-finding studies.
“We are delighted to launch the first program out of the PETT prioritization project, which has the potential to transform the multiple myeloma treatment paradigm…”
Also, lenalidomide’s protection on its composition of matter is set to expire by year’s end, and companies might begin marketing generic, cheaper versions of the medicine as early as 2022.”