- You are here:
- Home »
- Blog »
- non-conventional therapies »
- Stage l/ll Breast Cancer
Stage l/ll Breast Cancer
CONCLUSION-Tamoxifen at 5 mg/d for 3 years can halve the recurrence of breast intraepithelial neoplasia with a limited toxicity, which provides a new treatment option in these disorders…”
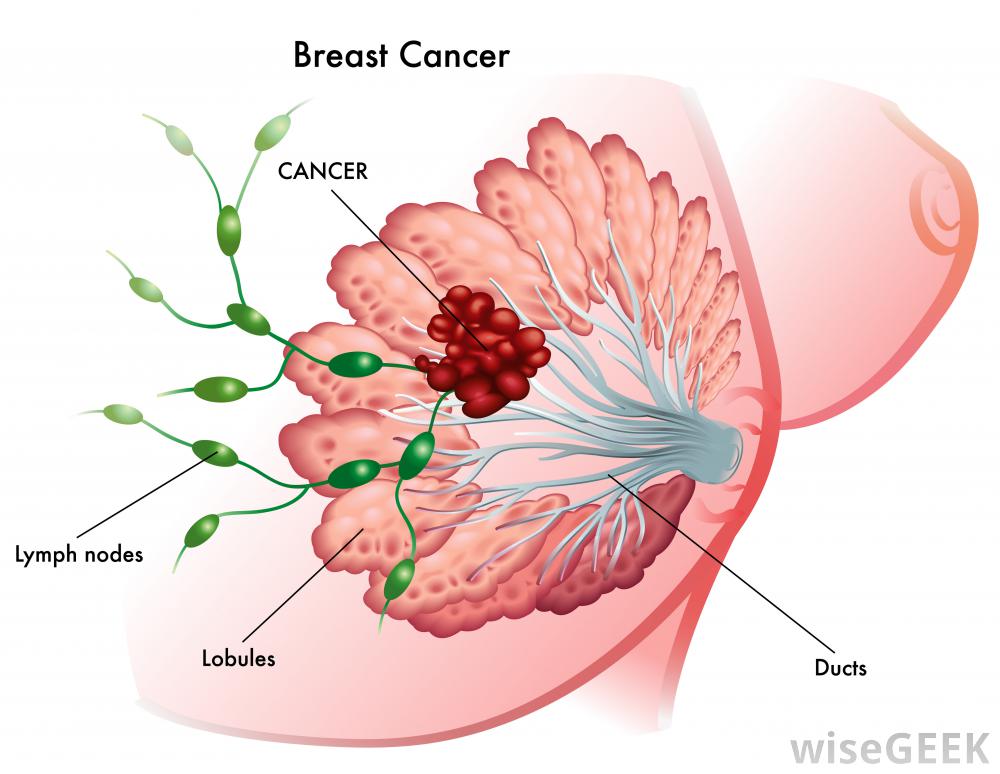
Dear David- I am a 60 yr old black woman in good health but was recently diagnosed with stage l/ll breast cancer. I am scheduled to have a lumpectomy in a week and at that time I will know if my lymph nodes have also been affected.
My question to you is the routine treatment for a lumpectomy is radiation therapy 3-6 weeks depending on my lymph node results but I am skeptical of receiving all that radiation.
My Dr. said not all women agree to radiation but do receive hormonal therapy for 1-5 years instead. Its been a bit overwhelming to say the least!!
- Cancer Survivor,
- Cancer Coach
- Director PeopleBeatingCancer
Recommended Reading:
Randomized Placebo Controlled Trial of Low-Dose Tamoxifen to Prevent Local and Contralateral Recurrence in Breast Intraepithelial Neoplasia
“PURPOSE– Tamoxifen administered for 5 years at 20 mg/d is effective in breast cancer treatment and prevention, but toxicity has limited its broad use. Biomarker trials showed that 5 mg/d is not inferior to 20 mg/d in decreasing breast cancer proliferation. We hypothesized that a lower dose given for a shorter period could be as effective in preventing recurrence from breast intraepithelial neoplasia but have a lower toxicity than the standard dose.
PATIENTS AND METHODS- We conducted a multicenter randomized trial of tamoxifen, 5 mg/d or placebo administered for 3 years after surgery in women with hormone-sensitive or unknown breast intraepithelial neoplasia, including atypical ductal hyperplasia and lobular or ductal carcinoma in situ. The primary end point was the incidence of invasive breast cancer or ductal carcinoma in situ.
RESULTS- Five hundred women 75 years of age or younger were included. After a median follow-up of 5.1 years (interquartile range, 3.9-6.3 years), there were 14 neoplastic events with tamoxifen and 28 with placebo (11.6 v 23.9 per 1,000 person-years; hazard ratio, 0.48; 95% CI, 0.26 to 0.92; P = .02), which resulted in a 5-year number needed to treat of 22 (95% CI, 20 to 27).
Tamoxifen decreased contralateral breast events by 75% (three v 12 events; hazard ratio, 0.25; 95% CI, 0.07 to 0.88; P = .02). Patient-reported outcomes were not different between arms except for a slight increase in frequency of daily hot flashes with tamoxifen (P = .02).
There were 12 serious adverse events with tamoxifen and 16 with placebo, including one deep vein thrombosis and one stage I endometrial cancer with tamoxifen and one pulmonary embolism with placebo.
CONCLUSION-Tamoxifen at 5 mg/d for 3 years can halve the recurrence of breast intraepithelial neoplasia with a limited toxicity, which provides a new treatment option in these disorders…”

